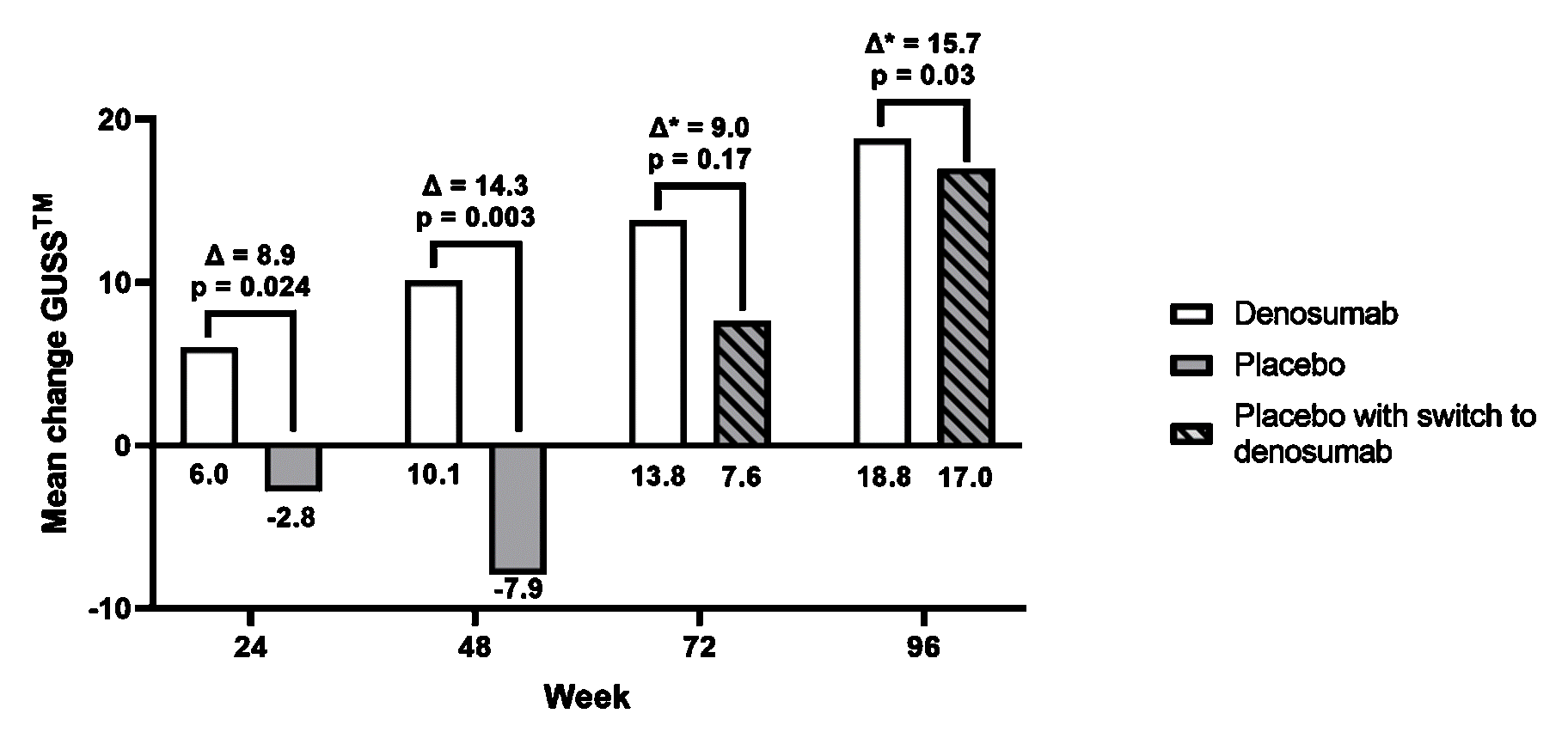Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Erosive hand osteoarthritis (OA) is a disabling disease with limited therapeutic options. Denosumab, a Receptor Activator of Nuclear Factor kappa-b Ligand inhibitor, affects bone resorption and osteoclast activity. The purpose of this clinical trial was to study the structure modifying effect of denosumab in patients with erosive hand OA, and to explore the clinical benefits and safety of subcutaneous denosumab 60 mg every 3 months versus placebo.
Methods: One hundred patients with erosive hand OA were randomly allocated to placebo or denosumab in a monocentric clinical trial lasting 48 weeks, followed by an open-label extension phase through week 96. The primary radiographic endpoint was change in total Ghent University Scoring System (GUSS™) score at week 24. GUSS™ (0-300) is a semi-quantitative scoring system specifically developed to combine scoring of both aspects of radiographic changes in erosive hand OA, being erosive progression (decrease of score) and signs of repair (increase of score) (1) and able to detect changes on short term (2). The secondary endpoint was the percentage of new erosive joints at week 48. Exploratory imaging (ultrasound) and clinical outcomes (pain, tender joint count, swollen joint count, grip strength, the Australian-Canadian Hand Osteoarthritis Index and the Functional Index for Hand Osteoarthritis) were assessed. Radiographic and clinical changes after 96 weeks of treatments were measured. Safety outcomes including (serious) adverse events, laboratory changes and changes in bone mineral density by dual-energy X-ray absorptiometry were registered. Primary efficacy analyses were performed in an intention-to-treat approach. Changes in GUSS™ were analysed at joint level with generalized estimating equations, accounting for within-patient clustering. Robust standard errors were used and the working correlation structure specified exchangeable Trial registration number: EUDRACT CT 2015-003223-53.
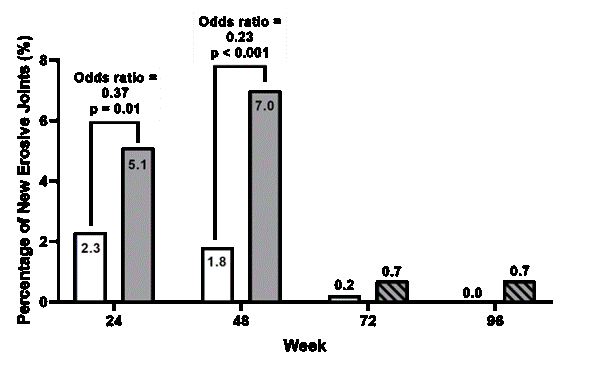
Results: 51 and 49 patients received subcutaneous administration of denosumab and placebo respectively. Total change GUSS™ was found statistically higher in denosumab compared to placebo at week 24 (mean change GUSS™ = 8.9 (95% CI: 1.0 – 16.9; p = 0.024))(Figure 1). This difference further increased at week 48 (ΔGUSS™ = 14.3 (95% CI: 4.6 – 24.0; p = 0.003)). Development of new erosive joints was significantly lower in denosumab (1.8%) compared to placebo (7.0%) at week 48 (OR = 0.23 (95% CI: 0.10 to 0.50); p < 0.001) (Figure 2). After open-label treatment through week 96, both groups continued remodelling and both pain and function significantly improved compared to baseline. No difference in occurrence of adverse events were observed between both groups.
Conclusion: Denosumab has clear structure modifying effects in erosive hand OA compared to placebo: significantly less erosive progression occurs after 24 weeks treatment, and the treatment effect enhances after 48 weeks. Symptom improvement was observed after sustained treatment.
References:
1. Verbruggen G et al. Ann Rheum Dis. 2010 May;69(5):862-7
2. Verbruggen G et al. Ann Rheum Dis. 2012 Jun;71(6):891-8
To cite this abstract in AMA style:
Wittoek R, Verbruggen A, Vanhaverbeke T, Elewaut D. Effect of Denosumab on Structure Modification in Erosive Hand Osteoarthritis: Results of a 48-Week, Monocentric, Randomized, Placebo-controlled, Double-blind Phase 2 Study and Open Label Extension Phase [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effect-of-denosumab-on-structure-modification-in-erosive-hand-osteoarthritis-results-of-a-48-week-monocentric-randomized-placebo-controlled-double-blind-phase-2-study-and-open-label-extension-pha/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-denosumab-on-structure-modification-in-erosive-hand-osteoarthritis-results-of-a-48-week-monocentric-randomized-placebo-controlled-double-blind-phase-2-study-and-open-label-extension-pha/