Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The presence of anti-topoisomerase I antibody (ATA) in patients with systemic sclerosis (SSc) has been associated with a greater risk of developing interstitial lung disease (ILD) and a greater rate of lung function decline in patients with early SSc. In the SENSCIS trial, nintedanib reduced the annual rate of decline in forced vital capacity (FVC) versus placebo in patients with SSc-ILD. We analyzed data from the SENSCIS trial in subgroups based on ATA status at baseline.
Methods: Patients with SSc according to the 2013 ACR/EULAR classification criteria, with ≥10% fibrosis of the lungs on a high-resolution computed tomography (HRCT) scan and with onset of the first non-Raynaud symptom < 7 years before screening were randomized to receive nintedanib 150 mg bid or placebo, stratified by the presence of ATA (based on historical information or, if not available, analysis at a central laboratory). We analyzed outcomes and adverse events over 52 weeks in subgroups of patients who were ATA positive or negative at baseline.
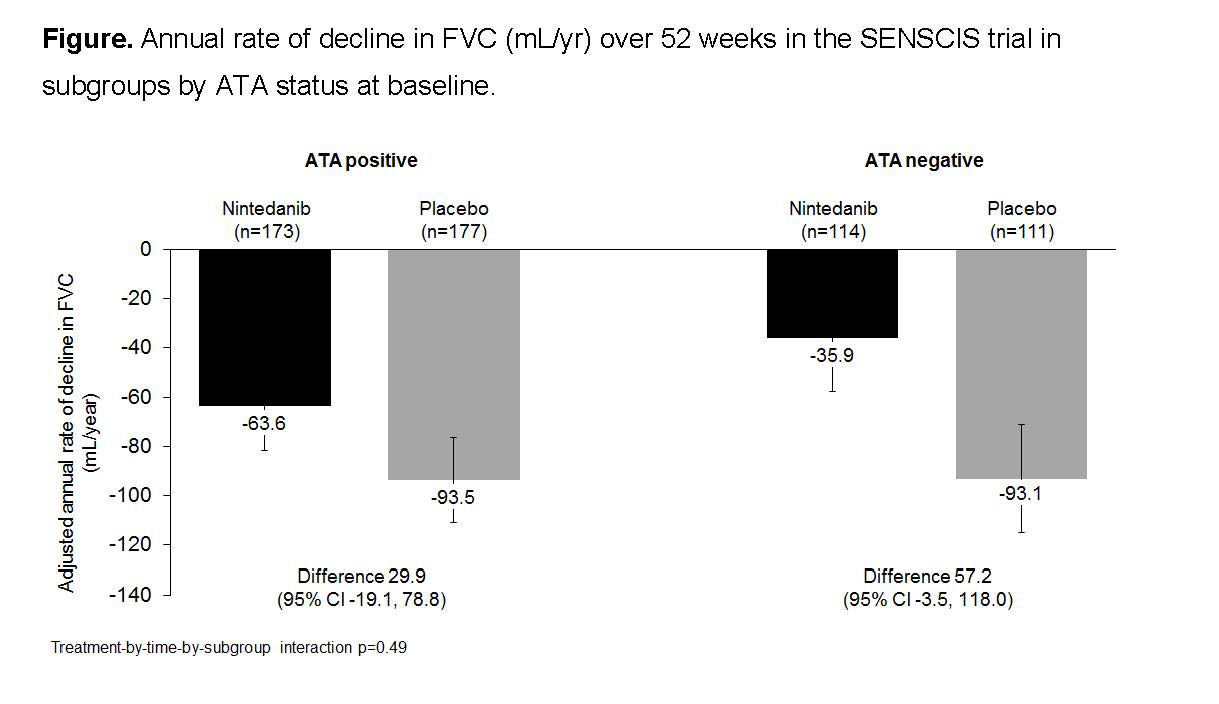
Results: Of 576 patients treated in the SENSCIS trial, 173 (60.1%) and 177 (61.5%) patients in the nintedanib and placebo groups, respectively, were ATA positive. In the subgroups that were ATA positive and negative, respectively, mean (SD) FVC (mL) was 2459 (773) and 2563 (781), and mean FVC % predicted was 71.4 (15.9) and 74.3 (17.7). Nintedanib reduced the rate of FVC decline compared with placebo both in patients who were ATA positive and negative. The treatment effect of nintedanib on reducing the rate of FVC decline was numerically greater in patients who were ATA negative than positive (Figure) (-63.6 vs -35.9 ml/year), but the treatment-by-time-by-subgroup interaction did not indicate heterogeneous treatment effects between the subgroups (p=0.49). In the nintedanib and placebo groups, respectively, absolute declines in FVC >5% predicted were seen in 23.1% and 30.5% of patients who were ATA positive (OR 0.69 [95% CI 0.43, 1.10]) and 16.7% and 25.2% who were ATA negative (OR 0.59 [0.31, 1.14]) (treatment-by-subgroup interaction p=0.73). Adjusted mean absolute changes from baseline in modified Rodnan skin score at week 52 in the nintedanib and placebo groups were -1.5 and -1.7 in patients who were ATA positive (difference 0.2 [95% CI -0.7, 1.2]) and -3.2 and -2.4 in patients who were ATA negative (difference -0.8 [-2.0, 0.4]; treatment-by-visit-by-subgroup interaction p=0.18). The adverse event profile of nintedanib was consistent between patients who were ATA positive and negative.
Conclusion: In the SENSCIS trial in patients with SSc-ILD, the rate of FVC decline over 52 weeks in placebo-treated patients was similar between patients who were ATA positive and ATA negative. Nintedanib reduced the rate of FVC decline compared with placebo both in patients who were ATA positive and negative, with a numerically greater treatment effect in patients who were ATA negative.
To cite this abstract in AMA style:
Mayes M, Highland K, Gahlemann M, Fischer A, Raghu G, Girard M, Alves M, Stowasser S, Distler J, Matucci-Cerinic M, Volkmann E, Kuwana M, Distler O. Effect of Anti-Topoisomerase I Antibody Status on Decline in Lung Function in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease: Data from the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effect-of-anti-topoisomerase-i-antibody-status-on-decline-in-lung-function-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-data-from-the-senscis-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-anti-topoisomerase-i-antibody-status-on-decline-in-lung-function-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-data-from-the-senscis-trial/