Session Information
Date: Saturday, November 6, 2021
Title: Health Services Research Poster I: Lupus, Inflammatory Arthritis, & More (0128–0148)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The clinical burden of systemic sclerosis (SSc) is substantial and typically characterized by progressive skin, gastrointestinal, pulmonary, and cardiovascular complications and premature death. The objective of this analysis was to describe the healthcare resource use (HCRU) and costs of SSc patients prior to and immediately after diagnosis.
Methods: A retrospective cohort comparison was performed using a claims dataset (IBM® MarketScan® Commercial Database) capturing beneficiary-level claims between 2015-19. SSc patients were identified using diagnosis codes for SSc. Eligible subjects had > 24 months of enrollment without a SSc diagnosis before their first SSc claim (‘index date’) and > 12 months of enrollment thereafter. Total HCRU and costs, as well as mean cost by care setting for inpatient, outpatient, physician office (PO), and prescription drugs were reported in 2019 USD as mean per member per year (PMPY) amounts for 3 time intervals: 2- and 1-year before and 1-year after index diagnosis. To show the relative cost of SSc, patients with rheumatoid arthritis (RA) but not SSc and a general population (‘unaffected’) cohort without SSc or RA were matched 1:1 to SSc patients on age and gender.
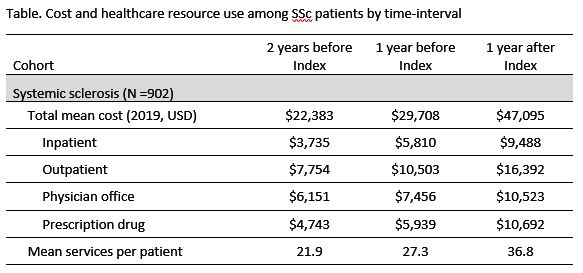
Results: 902 SSc patients were eligible. Mean age at index SSc diagnosis was 54.3 years old and 84.7% were female. Total mean PMPY cost increased each year from $22,383 to $29,708 to $47,095, 2 years before, 1-year before, and 1-year after index diagnosis, respectively. These costs were higher than the RA (by 17, 27, and 28%) and unaffected (by 119, 208, and 278%) cohorts. Among SSc patients, the outpatient setting represented the largest proportion of cost 1-year after index ($16,392), followed by prescription drugs ($10,692), PO ($10,523), and inpatient ($9,448) settings. PMPY cost for diagnostic imaging services increased each year from $807 to $1,383 to $1,779. 1-year after SSc diagnosis, mean PMPY costs for immunosuppressant, corticosteroid, endothelin receptor antagonist, and proton-pump inhibitor drugs were $4,183, $1,746, $814, and $783, respectively. Among SSc patients, mean healthcare services per patient increased each year from 22 to 27 to 37 services and were higher as compared to the RA (21, 26, and 32) and unaffected (13, 14, and 15) cohorts.
Conclusion: SSc patients appear to accrue markedly greater healthcare costs and require more services than a matched unaffected general population. Elevated expenditures and HCRU are observed from at least 2 years before a confirmed SSc diagnosis with both increasing over time, a dynamic that likely reflects both the progressive, multi-system nature of SSc and the potential challenges and delays in diagnosis. Additionally, despite no proven disease modifying therapy, SSc patients still accrued substantial drug costs for therapies likely used to suppress emerging sequalae of the underlying disease. Together these findings suggest that SSc patients pose a substantial burden on the US healthcare system and highlights the need for early diagnosis and effective therapies.
To cite this abstract in AMA style:
Khanna D, Furst D, Li J, Meng Q, Lesperance T, Peoples K, Ali F, LaMoreaux B, Taylor S. Economic and Healthcare Resource Use Burden of Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/economic-and-healthcare-resource-use-burden-of-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/economic-and-healthcare-resource-use-burden-of-systemic-sclerosis/