Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Raynaud’s phenomenon (RP) is often the initial clinical manifestation of systemic sclerosis (SSc). RP alerts clinicians to the possibility of an autoimmune rheumatic disease, prompting examination for cutaneous and musculoskeletal signs and antinuclear antibodies (ANA). However, some SSc patients develop other manifestations, particularly puffy hands, prior to the onset of RP, which can make early SSc more challenging to recognize. In order to help elucidate the diversity of SSc presentation in its early stages, we describe the initial clinical manifestations and ANA profiles of patients whose initial SSc manifestation was not RP in two early SSc cohorts.
Methods: Two independent SSc cohorts were examined, both of which enrolled patients within five years of the first non-RP symptom. Patients in cohort one (n = 450) met the 1980 ACR preliminary classification criteria or the 2013 ACR/EULAR classification criteria. Patients in cohort two (n = 938) met the 2013 ACR/EULAR classification criteria. The initial clinical manifestation of SSc, demographics, metrics of disease severity, and ANA results were determined and compared between those whose initial manifestation was RP vs. a non-RP symptom.
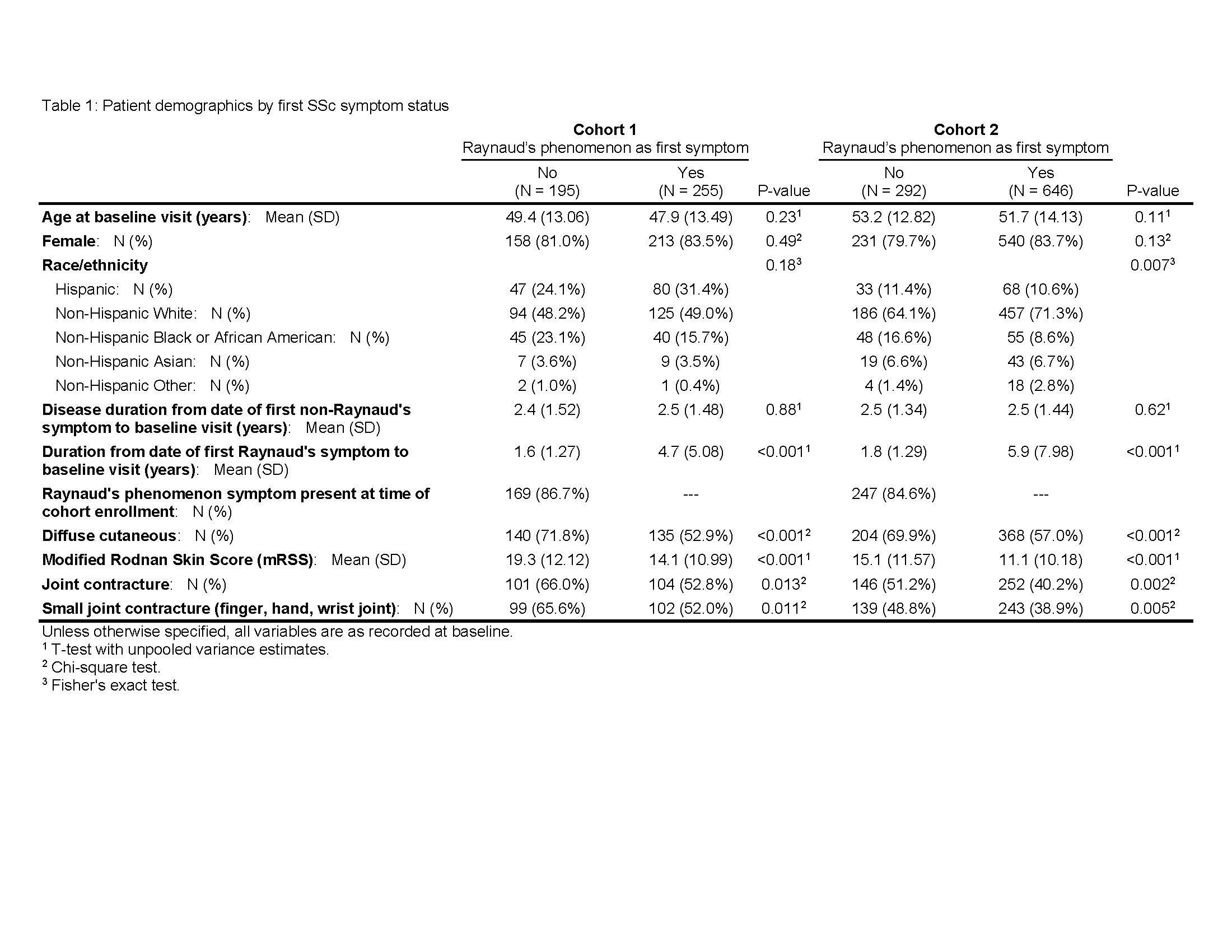
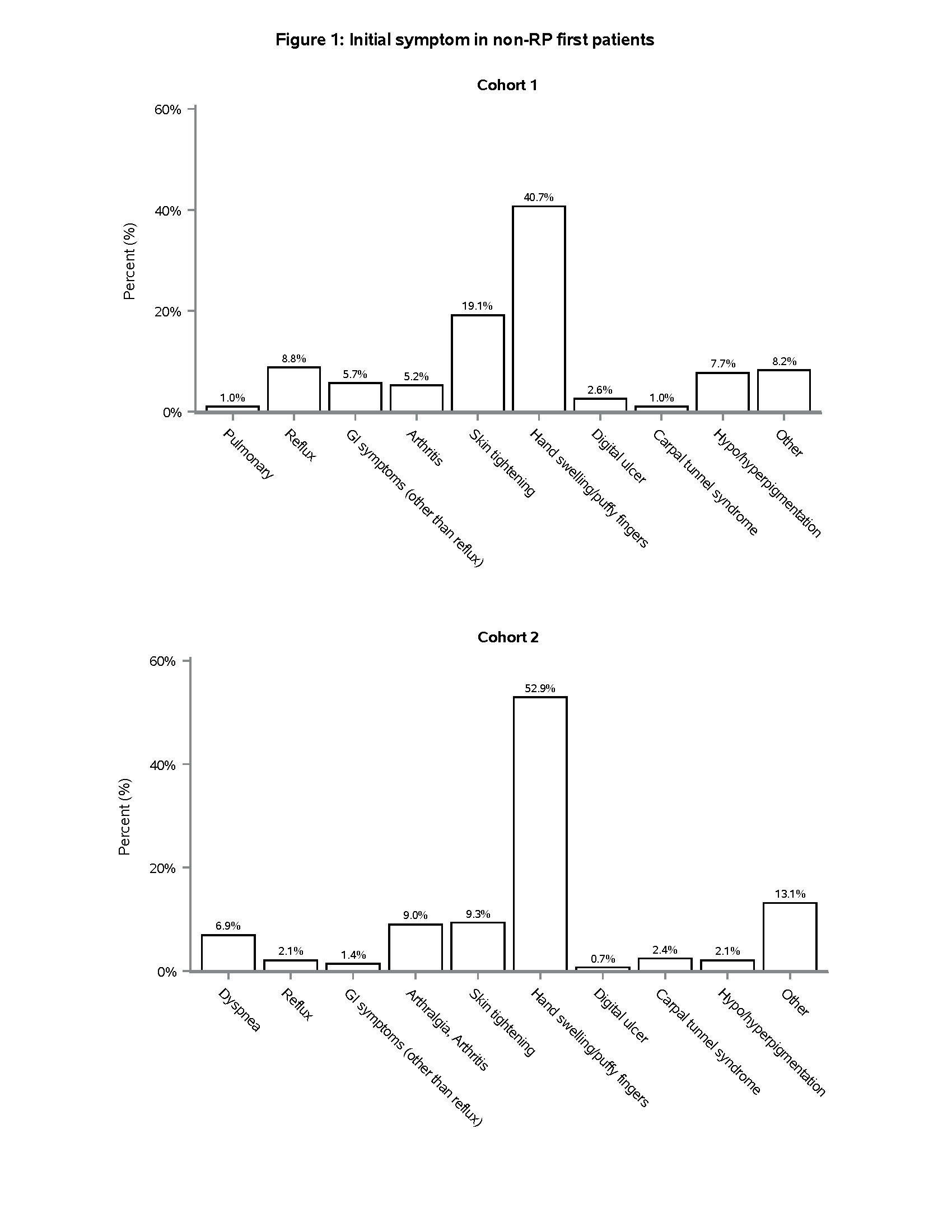
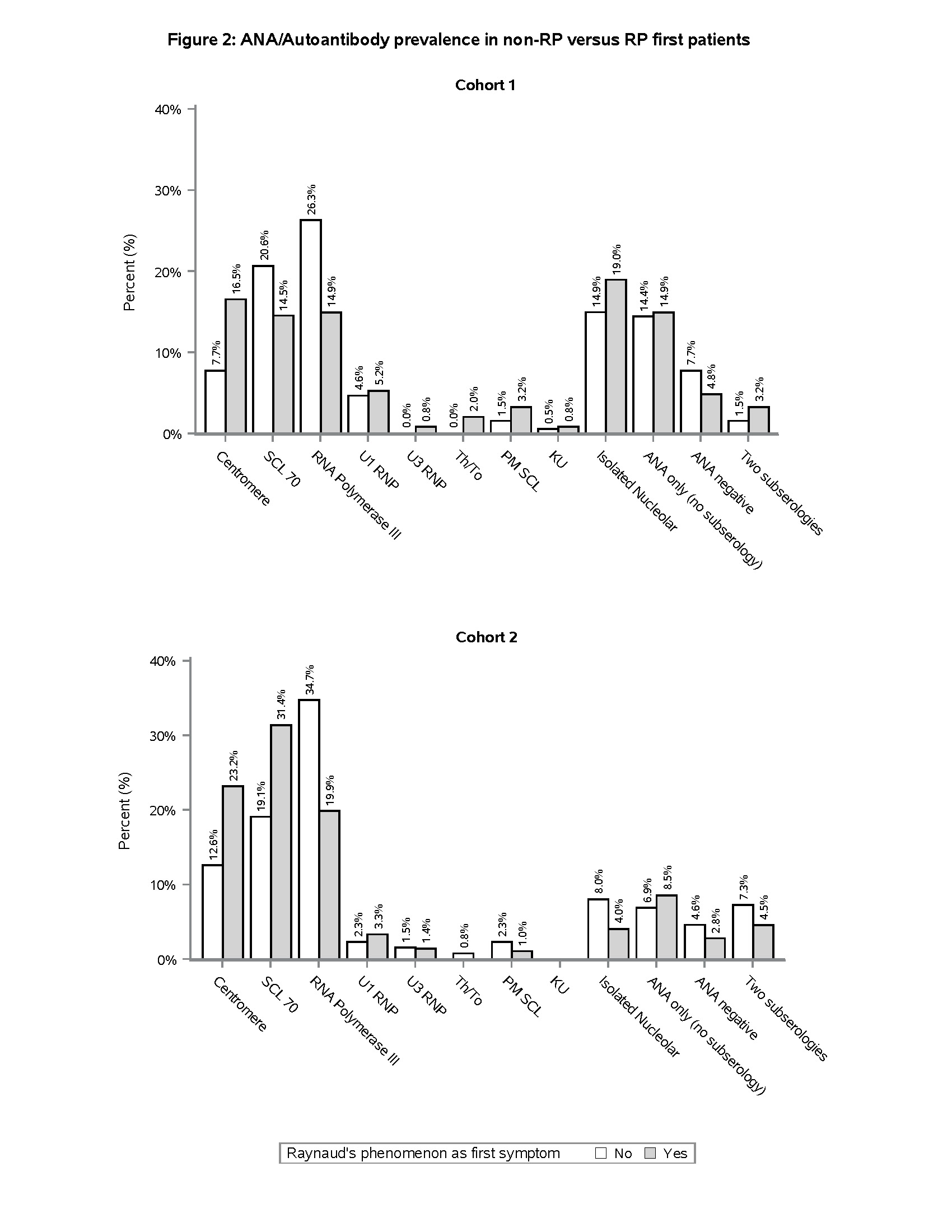
Results: 195/450 (43.3%), and 292/938 (31.1%) SSc patients in cohorts one and two, respectively, had a non-RP initial symptom (Table 1), the most common of which was puffy hands (Figure 1). Non-RP first presentation was more common amongst Black patients compared to other race/ethnicity categories (Table 1). Despite similar time from the first non-RP symptom to enrollment, non-RP first patients were more likely than RP first patients to have diffuse cutaneous involvement (71.8% vs. 52.9%, p < 0.001 in Cohort 1; 69.9% vs. 57.0%, p < 0.001 in Cohort 2), higher mean mRSS (19.3 vs. 14.1, p < 0.001 in Cohort 1; 15.1 vs. 11.1, p < 0.001 in Cohort 2), and joint contractures (66.0% vs. 52.8%, p = 0.013 in Cohort 1; 51.2% vs. 40.2%, p = 0.002 in Cohort 2) (Table 1). In both cohorts, RNA Polymerase III antibody was more prevalent in non-RP first compared to RP first patients (26.3% vs. 14.9%, p = 0.003 in Cohort 1; 34.7% vs. 19.9%, p < 0.001 in Cohort 2) (Figure 2). 26/450 (5.8%) and 45/938 (4.8%) patients in cohorts one and two, respectively, had not experienced RP at all at the time of enrollment. The majority of these patients had diffuse cutaneous involvement and joint contractures (data not shown).
Conclusion: In two independent cohorts, we found that >30% of SSc patients had begun to manifest SSc with puffy hands or other symptoms without the “warning sign” of RP as their initial symptom. These patients tended to present with more severe skin and joint disease, highlighting the importance of early recognition. The most common autoantibody associated with this presentation was RNA Polymerase III, which importantly is not included in some of the multiplex antibody panels often used as ANA screening tools in clinical practice. These results should be considered in efforts to recognize SSc in its earliest stages. Puffy hands, even in the absence of RP, should prompt consideration of early SSc and testing for ANA by an approach that is inclusive of RNA Polymerase III and other nucleolar antigens.
To cite this abstract in AMA style:
Hanif I, Assassi S, Mayes M, Zhang M, Charles J, VanBuren J, Alvey J, Ghaffari K, Bernstein E, Castelino F, Chung L, Evnin L, Frech T, Gordon J, Hant F, Hummers L, Khanna D, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Molitor J, Moore D, Richardson C, Sandorfi N, Shah A, Shah A, Shanmugam V, Steen V, Volkmann E, Zahn C, Skaug B. Early Scleroderma with Non-Raynaud’s Symptoms Prior to Raynaud’s Onset Is Associated with Rapid Progression to Diffuse Skin Disease and Joint Contractures [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/early-scleroderma-with-non-raynauds-symptoms-prior-to-raynauds-onset-is-associated-with-rapid-progression-to-diffuse-skin-disease-and-joint-contractures/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-scleroderma-with-non-raynauds-symptoms-prior-to-raynauds-onset-is-associated-with-rapid-progression-to-diffuse-skin-disease-and-joint-contractures/