Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Aberrant Neutrophil Extracellular Traps (NETs) production contributes to the pathophysiology of multiple inflammatory and autoimmune diseases such as Rheumatoid arthritis (RA) Hidradenitis suppurativa (HS), Systemic Lupus Erythematosus and ANCA-Associated Vasculitis. Here we report the first in human studies of a humanized monoclonal antibody (CIT-013) with high affinity for citrullinated histones H2A and H4. CIT-013 inhibits NET formation and accelerates the clearance of NETs and netting neutrophils to lower NET tissue burden in vivo with significant anti-inflammatory consequences in pre-clinical studies (Chirivi et al 2021; DOI 10.1038/s41423-020-0381-3).
The objectives of the studies were:
- Part A: to assess safety, tolerability, andpharmacokinetics (PK) of intravenous (IV) single ascending doses of CIT-013.
- Part B: to assess safety, tolerability, PK, and pharmacodynamics (PD) by inducing systemic NET formation by nano-dosing lipopolysaccharide (LPS) after single IV doses of CIT-013.
- Part C: to evaluate safety, tolerability, bioavailability, and PK of single subcutaneous (SC) doses of CIT-013.
Methods: The study was a randomized, double-blind, placebo-controlled, phase I study with a single dose of CIT-013 in healthy volunteers. In part A, single ascending doses of 0.1, 0.3, 1.0, 1.8 and 3.0 mg/kg CIT-013 or placebo were administered IV. In part B, 0.3 or 0.9 mg/kg CIT-013 or placebo were given IV before 2ng/kg LPS. LPS will trigger the innate immune system resulting in measurable circulating NET components in the blood. In part C, 50 or 100 mg CIT-013 or placebo was administrated SC. Endpoints were safety, tolerability, and PK in all three parts, plus PD in part B.
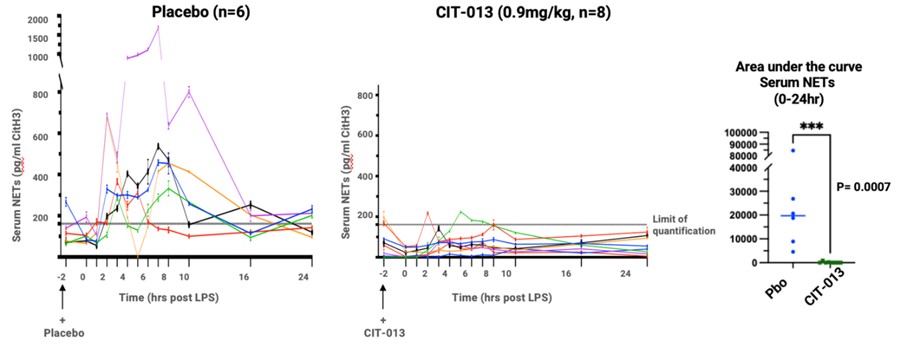
Results: In total 73 healthy volunteers were dosed (Figure 1) and no severe, or Serious Adverse Events (SAEs) were reported. In part A, IV administration of CIT-013 was well tolerated up to 0.3 mg/kg alone, and 0.9 mg/kg with premedication. Dose limiting toxicity was seen at doses greater than 0.9 mg/kg with chest discomfort associated with elevated cytokines, CRP and temperature as the most common AEs. CIT-013 given SC at 50 mg or 100 mg was well tolerated. The only AEs of note were mild injection site reactions. PK was consistent with what would be expected from an IgG1 human monoclonal antibody with a half-life of approximately 7 days. The bioavailability of 100 mg given SC was 66% compared to IV administration and equated to approximately 0.7 mg/kg given IV (Figure 2). CIT-013 markedly reduced LPS induced levels of systemic NET components, equally well at 0.3 and 0.9 mg/kg, demonstrating effective target engagement (Figure 3).
Conclusion: CIT-013 is well tolerated up to 0.3 mg/kg intravenously and up to 0.9 mg/kg with pre-medication. Dose limiting toxicity was seen when given intravenously, with chest discomfort associated with elevated cytokines, CRP and temperature which was markedly reduced by subcutaneous administration. Near complete inhibition of circulating NET components at 0.3 and 0.9 mg/kg CIT-013 in the LPS challenge study showed effective target engagement. These data form the basis for further development of CIT-013 in RA and HS.
To cite this abstract in AMA style:
Middelink L, Chirivi R, van Es H, Meldrum E, van Zandvoort P, Bruurmijn T, Moerland M, Round P. Early Clinical Development of CIT-013, a First in Class NETosis Inhibitor, in a Randomized Phase I Dose Escalation Study in Healthy Volunteers Demonstrating Potent Inhibition of LPS Induced Neutrophil Extracellular Trap Formation [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/early-clinical-development-of-cit-013-a-first-in-class-netosis-inhibitor-in-a-randomized-phase-i-dose-escalation-study-in-healthy-volunteers-demonstrating-potent-inhibition-of-lps-induced-neutrophil/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-clinical-development-of-cit-013-a-first-in-class-netosis-inhibitor-in-a-randomized-phase-i-dose-escalation-study-in-healthy-volunteers-demonstrating-potent-inhibition-of-lps-induced-neutrophil/