Session Information
Date: Monday, October 27, 2025
Session Type: Abstract Session
Session Time: 3:15PM-3:30PM
Background/Purpose: Fatigue is a pervasive and burdensome symptom for people with psoriatic arthritis (PsA). The mechanisms underlying PsA related fatigue are unknown. In other clinical populations, the brain’s insula region, a key integration hub between peripheral signals of pain/inflammation and central signals such as mood, has emerged as a key component of the fatigue network1. Moreover, excess levels of brain glutamate has been implicated with this symptom2. To date, no studies have specifically examined the potential role of brain connectivity and glutamate in PsA fatigue.
Methods: Patients with active PsA who fulfilled the CASPAR criteria were evaluated. Fatigue was measured using a fatigue numeric rating scale averaged over the prior week (7-day fatigue NRS). Subjects then undertook functional MRI and single voxel MRI spectroscopy brain scans. Images were acquired using 3 Tesla Siemens PRISMA with 32-channel phased array head coil using a T2*-weighted gradient-echo echo-planar imaging pulse sequence. Functional MRI data was analyzed using functional connectivity CONN toolbox employing a right posterior insula seed. The functional connectivity of this region of interest to the whole brain was estimated, followed by group level multiple linear regression to 7-day fatigue NRS corrected for age, sex and multiple comparisons (FDR< 0.05). Right posterior insula spectroscopy data was analyzed using LCModel. Individual glutamate/glutamine concentrations were calculated and corrected for levels of brain creatine (Glx/Cr). Glx/Cr correlation with fatigue scores and insula seed functional connectivity was assessed.
Results: 38 enrolled patients completed both functional MRI and MRI spectroscopy (mean age 46.7 ± 9.9; 47% female; mean BMI 29.2 ± 4.6; mean 7-day fatigue NRS 6.4 ± 2.8; mean DAPSA 38 ± 15.8). Higher fatigue scores correlated with higher functional connectivity from the right posterior insula to cerebellum (R=0.694, p< 0.022FDR) and anti-correlated with functional connectivity to the superior frontal gyrus, which covers the medial prefrontal cortex (R=-0.7029, p=0.0008FDR) (Figure A). PsA patients with higher fatigue scores also trended towards increased Glx/Cr (R=0.3, p=0.07) in the right posterior insula and these levels significantly correlated with fatigue associated functional connectivity of this region (cerebellum: R=0.49, p=0.003 and superior frontal gyrus: R=-0.4, p=0.016) (Figure B).
Conclusion: We demonstrate for the first time that the brain may have a role in PsA fatigue. Specifically, the multifaceted role of the insula is highlighted. Altered functional connectivity between the posterior insula to brain regions involved in movement, motivation, and peripheral sensations (cerebellum); and executive decision making/emotions (superior frontal gyrus) appears to characterize fatigue severity in PsA. Excess of the excitatory neurotransmitter glutamate within the posterior insula may mediate this neural dysfunction but further research is warranted to understand it’s functional role and clarify its potential as a future therapeutic target for PsA fatigue.
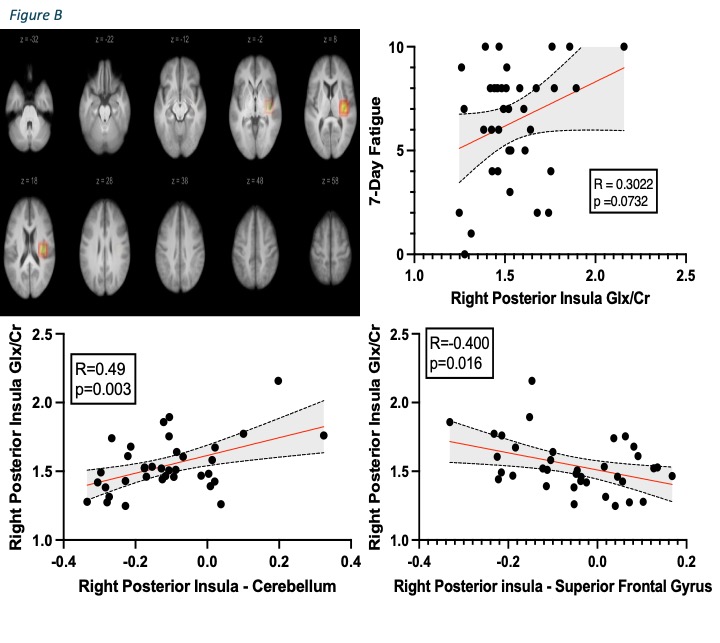 Figure A shows significant altered functional connectivity that correlates with fatigue scores. The top panel demonstrates the insula seed used for the seed-to-voxel analysis. The images show the voxel positions (colored area on brain slice) with slice number (Z) at the top of each image. The scatterplots visualize the correlation of functional connectivity (Fischer z-transformed r values) between two brain regions and fatigue scores, Pearsons R, and p values after false discovery rate for multiple comparisons (FDR). The tables demonstrate results from multi-linear modelling (correcting for age and sex), degrees of freedom (df), peak voxel co-ordinates in MNI space (x,y,z), and cluster size.
Figure A shows significant altered functional connectivity that correlates with fatigue scores. The top panel demonstrates the insula seed used for the seed-to-voxel analysis. The images show the voxel positions (colored area on brain slice) with slice number (Z) at the top of each image. The scatterplots visualize the correlation of functional connectivity (Fischer z-transformed r values) between two brain regions and fatigue scores, Pearsons R, and p values after false discovery rate for multiple comparisons (FDR). The tables demonstrate results from multi-linear modelling (correcting for age and sex), degrees of freedom (df), peak voxel co-ordinates in MNI space (x,y,z), and cluster size.
.jpg) Figure B shows (top left) the average position across all participants of 20x20x20mm voxel for the right posterior insula used in this analysis. The top right figure demonstrates right posterior insula glutamate/glutamine levels correlation of creatine (Glx/Cr) with 7-day fatigue scores. Bottom scatterplots illustrate right posterior insula functional connectivity correlating with right posterior insula Glx/Cr levels.
Figure B shows (top left) the average position across all participants of 20x20x20mm voxel for the right posterior insula used in this analysis. The top right figure demonstrates right posterior insula glutamate/glutamine levels correlation of creatine (Glx/Cr) with 7-day fatigue scores. Bottom scatterplots illustrate right posterior insula functional connectivity correlating with right posterior insula Glx/Cr levels.
To cite this abstract in AMA style:
McGucken A, Sunzini F, Stefanov K, Parkinson J, Al-Wasity S, McLean J, Waiter G, Siebert S, Basu N. Dysfunctional Neurobiology Of The Insula Characterizes Fatigue In Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/dysfunctional-neurobiology-of-the-insula-characterizes-fatigue-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dysfunctional-neurobiology-of-the-insula-characterizes-fatigue-in-psoriatic-arthritis/
