Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Linking inflammation to fibrosis, a common end stage feature of many autoantibody mediated rheumatic diseases, remains a challenge. Indeed the signature hallmark of anti-Ro associated congenital heart block (CHB) is rapid replacement of the AV node by fibrosis during the late 2nd trimester. Previously reported in vitro coculture experiments and transcriptomic analysis position type I Interferons in the pathogenesis of conduction disease, but it remains to be identified how IFNa contributes to a pathological cardiac fibroblast. By focusing solely on smooth muscle actin (SMAc), heterogeneity in myofibroblasts has been underappreciated. The current study was initiated to evaluate Fibroblast-Specific Protein 1 (FSP1), a fibroblast marker with demonstrated responsiveness to the Wnt/????-catenin pathway, and its contribution, in concert with interferon alpha (IFNa), to cardiac injury propagated by maternal anti-Ro.
Methods: An in vitro model of anti-Ro mediated CHB employed co-culture of supernatants by hY3 (a noncoding ssRNA, anti-Ro proxy)-treated human macrophages (THP1 cells) with human cardiac fetal fibroblasts. In addition, fibroblasts were treated with IFNa or recombinant Wnt3A with and without their respective inhibitors (neutralizing IFNa antibody (NIFNa), ICG-001). Using standard techniques, assessments included transcriptomics (qPCR, IFNa stimulated genes (ISG)), immunofluorescence (IF) and flow cytometry (FC).
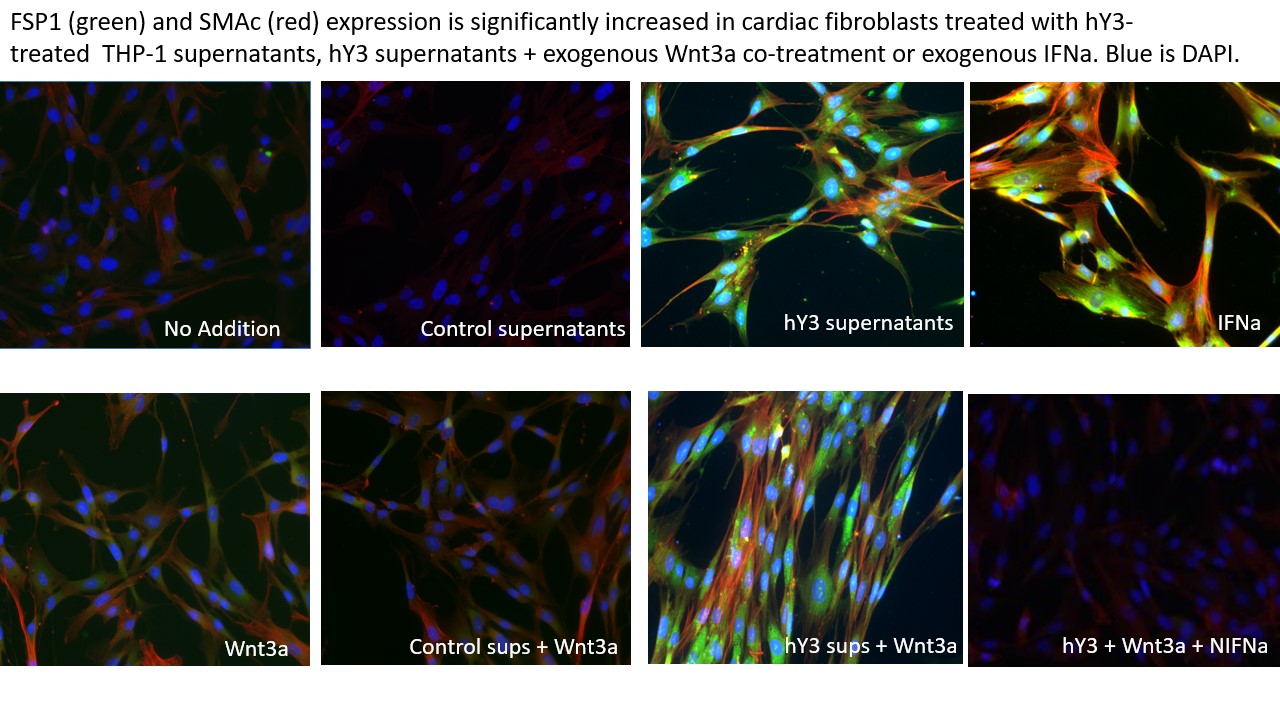
Results: Use of derivative of hY3-transfected THP1 cells (hY3 supernatants) markedly upregulated the fibroblast expression of ISGs, IFIT-1 and SIGLEC-1 supporting the secretion of IFNa by the THP-1 cells. After exposure to IFNa, SMAc and FSP1 expression in fibroblasts were significantly increased compared to untreated cells (SMAc: 23,347±2077 vs. 89.5±21, p< 0.01; FSP1: 2282.7±293 vs. 70.1±3, N=3, p< 0.01). As predicted, fibroblasts treated with hY3 supernatants also significantly promoted SMAc and FSP1 myofibroblast subtypes compared to untreated fibroblasts using IF (Figure 1) and FC (SMAc: 6463.7±593 vs. 89.5±21, N=3, p< 0.01; FSP: 1339.0±154 vs. 70.1±3, N=3, p< 0.02) but to a lesser degree than observed with IFNa . There was a gap in SMAc and FSP1 expression between IFNa and hY3 treatments, however, it was mitigated when fibroblasts were co-treated with hY3 supernatants and Wnt3a. hY3 supernatants/Wnt3a co-treatment showed remarkable synergy, unexplained by either treatment alone (hY3 supernatant + Wnt3a vs IFNa-induced expression (SMAc: 20,526.3±2617 vs. 23,346.7±2077, N=3, n.s., FSP1: 2118.7±193 vs. 2282.7±293, N=3, n.s.)., a phenotype that was attenuated by cotreatment with NIFNa, a result implicating IFNa as an autacoid factor of dual fibroblast transdifferentiation (IF, Figure 1, lower right panel). In parallel, the contribution of Wnt3a to IFNa a-induced FSP1 and SMAc expression was supported by their reduced expression when IFNa -treated fibroblasts were exposed to ICG-001.
Conclusion: These data support that an important component of anti-Ro mediated injury involves pathological activated fibroblast phenotypes reflecting dual transdifferentiation mediated by type I Interferon and disorders of the Wnt/????-catenin pathway.
To cite this abstract in AMA style:
Firl C, Chang M, Buyon J, Clancy R. Dual Fibroblast Transdifferentiation Mediated by Type I Interferon: Application to Anti-Ro Mediated Congenital Heart Block [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/dual-fibroblast-transdifferentiation-mediated-by-type-i-interferon-application-to-anti-ro-mediated-congenital-heart-block/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dual-fibroblast-transdifferentiation-mediated-by-type-i-interferon-application-to-anti-ro-mediated-congenital-heart-block/