Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Oxidative stress plays a role in the progression of inflammatory diseases such as Rheumatoid Arthritis (RA) due to the imbalance between levels of reactive oxygen species (ROS) and endogenous antioxidant defense mechanisms. Resulting from oxidative stress, Malondiadehyde-Acetaldehyde protein adduct (MAA) formation is increased in RA joint tissues where these co-localize with citrullinated proteins, acting as potent immune adjuvants. Doxycycline (DOX) and other tetracycline derivatives demonstrate efficacy in the treatment of RA. Interestingly, this clinical benefit does not appear to stem from their antibacterial properties, and the anti-inflammatory properties of DOX are currently unknown. Therefore, we tested the hypothesis that DOX directly scavenges ROS and inhibits the formation of redox-mediated MAA-adduct formation.
Methods: To test whether DOX inhibits the formation of MAA-Albumin, a cell-free system was used by adding DOX to human serum albumin (ALB) in the presence of 2mM malondiadehyde (MDA) and 1mM acetaldehyde (AA). MAA-ALB formation was monitored by autofluorescence at 498 nm. Electron paramagnetic resonance (EPR) was used to quantify free radical production and superoxide scavenging in the presence/absence of DOX. HEK 293 cells transfected with the nuclear factor erythroid 2-related factor/antioxidant response element (Nrf2/ARE) were used to determine whether DOX alters intracellular redox signaling pathways activated by MAA-adduct generation. Nrf2 activation was quantified by measuring the amount of luciferase via cellular luminescence.
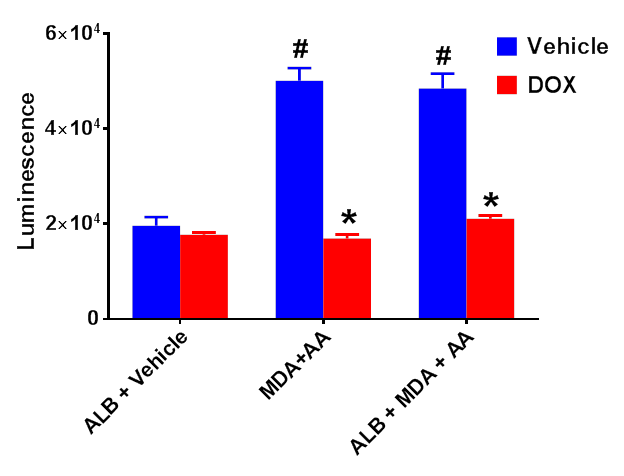
Results: Cell-free studies showed that DOX reduced MAA-adduct formation at all time points investigated (p<0.0001). EPR revealed that DOX significantly inhibited free radical production related to MAA-adduct formation and scavenged superoxide (p<0.0001 vs. sham). Finally, incubation of AA, MDA and/or ALB increased Nrf2 activation (Figure 1), an effect that was significantly reduced (p<0.001) in the presence of DOX.
Conclusion: DOX, a drug used sparingly in the management of RA and other chronic inflammatory diseases, scavenges ROS produced in the process of MAA-adduct formation. Additionally, DOX inhibits MAA-induced Nrf2 activation, a redox-sensitive transcription factor, thus demonstrating its ability to alter intracellular redox signaling. Together, these data strongly indicate a novel antioxidant property of DOX that could explain its clinical benefit in the context of select chronic inflammatory conditions that are characterized by oxidative stress.
Figure 1.
To cite this abstract in AMA style:
Chiou A, Duryee MJ, Clemens DL, Sarmiento C, Zimmerman M, Hunter CD, Klassen LW, O'Dell JR, Anderson DR, Mikuls TR, Thiele GM. Doxycycline Directly Scavenges Reactive Oxygen Species and Inhibits the Formation of Malondialdehyde-Acetaldehyde-Protein Adducts – a Novel Mechanism of Action for an Old Drug [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/doxycycline-directly-scavenges-reactive-oxygen-species-and-inhibits-the-formation-of-malondialdehyde-acetaldehyde-protein-adducts-a-novel-mechanism-of-action-for-an-old-drug/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/doxycycline-directly-scavenges-reactive-oxygen-species-and-inhibits-the-formation-of-malondialdehyde-acetaldehyde-protein-adducts-a-novel-mechanism-of-action-for-an-old-drug/