Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In the US, the recommended starting dose of intravenous tocilizumab (TCZ) in combination with DMARDs or as monotherapy is 4 mg/kg every 4 weeks and increased to 8 mg/kg based on clinical response for patients with moderate to severe rheumatoid arthritis (RA)1; however, data on actual dose escalation patterns are limited. The objective of this analysis was to evaluate how intravenous TCZ is dosed in a real-world setting using data from the Corrona registry.
Methods: All patients enrolled in the comparative effectiveness substudy (CERTAIN) nested within Corrona who initiated TCZ and had 3- and 6-month follow-up data available were eligible for inclusion in this analysis. Data were collected from patients who remained on their initial TCZ dose of 4 mg/kg at 3 months (Group 1) and patients who had their TCZ dose escalated to 8 mg/kg by or at 3 months (Group 2). Unadjusted efficacy response data were provided for patients at 3 and 6 months, including Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP), Clinical Disease Activity Index (CDAI), CRP, modified Health Assessment Questionnaire, patient pain, patient fatigue and EULAR response.
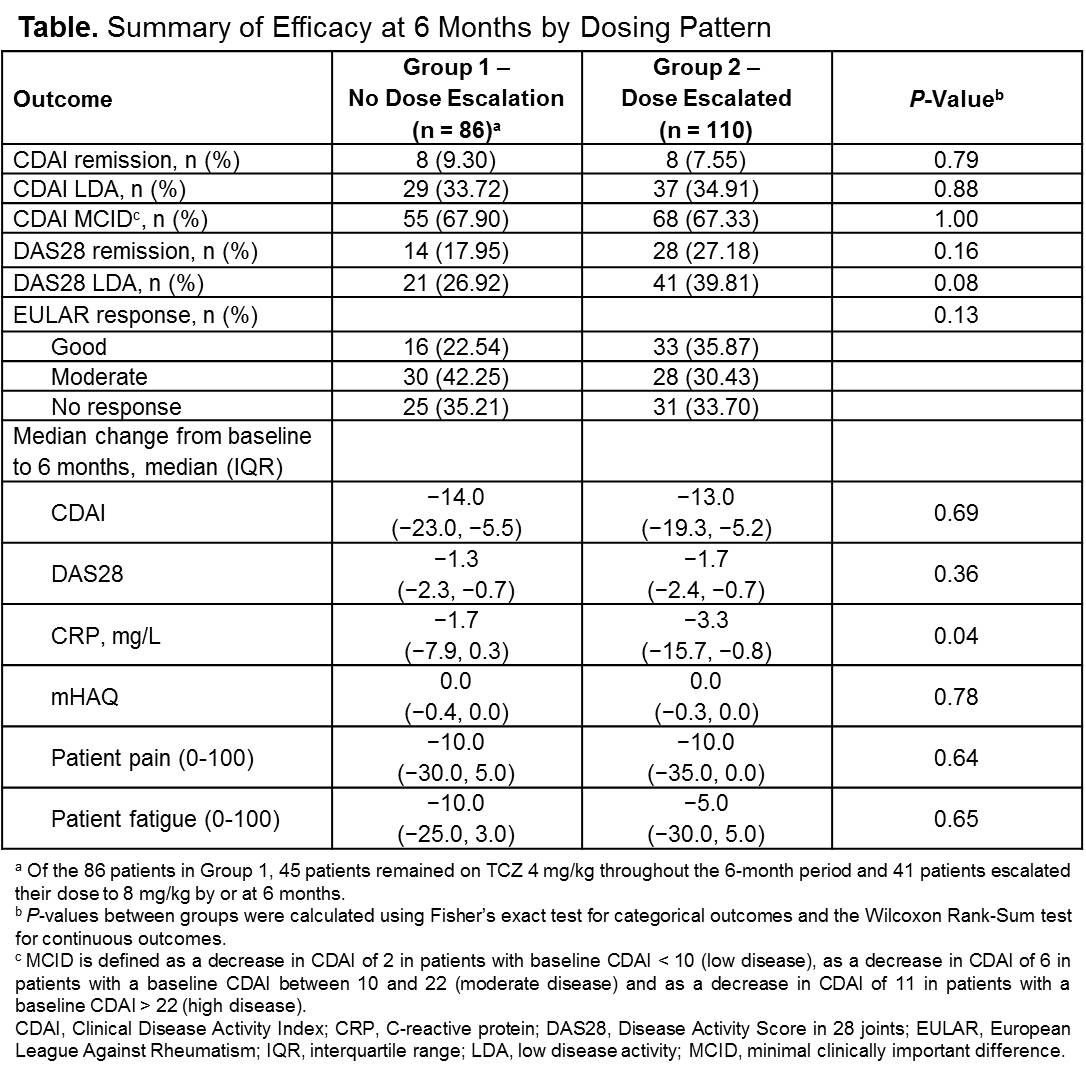
Results: Of the 196 patients included in this analysis, 56.1% (95% CI, 48.9% to 63.2%) escalated their TCZ dose by or at 3 months (Group 1, n = 86; Group 2, n = 110). Baseline characteristics were similar between dosing groups, except patients in Group 2 were older (58.3 vs 54.0 years) and a lower proportion were female (75.5% vs 89.4%) compared with patients in Group 1. At baseline, the mean (SD) CDAI was 32.4 (13.9) and 31.3 (12.9), mean (SD) DAS28-CRP was 5.1 (1.1) and 5.0 (1.2) and mean (SD) CRP was 10.4 (15.1) mg/L and 13.0 (20.2) mg/L in Group 1 and Group 2, respectively. The median (IQR) number of prior biologics was 2.0 (1.0, 4.0) and 2.0 (1.0, 3.0) in Group 1 and Group 2, respectively, suggesting a rather refractory disease. Unadjusted disease activity response outcomes at 3 months were mostly similar between the 2 groups, except median (IQR) decrease from baseline in CRP was significantly greater in Group 2 vs Group 1 (-3.4 [-12.8, -0.8] mg/L vs -1.0 [-5.0, 1.0] mg/L; P = 0.001). At 6 months, efficacy outcomes remained similar between the 2 groups (Table); however, a numerically higher proportion of patients in Group 2 achieved EULAR good responses, CDAI low disease activity and DAS28-CRP low disease activity and remission vs Group 1, and median decrease from baseline to 6 months in CRP was significantly greater in Group 2 vs Group 1.
Conclusion: Real-world data show that physicians escalate the dose of TCZ at varying frequencies. In slightly more than one half of TCZ initiators in this refractory to therapy population, the TCZ dose was escalated by 3 months. The majority of patients in both groups achieved moderate or good EULAR response.
Reference:
1. ACTEMRA [Highlights of Prescribing Information]. South San Francisco, CA: Genentech, Inc; 2013.
Disclosure:
D. A. Pappas,
Corrona, LLC,
3,
Novartis,
9;
A. John,
Genentech, Inc,
3;
J. R. Curtis,
AbbVie, Amgen, BMS, Corrona, LLC, Crescendo, Janssen, Pfizer, Roche/Genentech and UCB,
5,
AbbVie, Amgen, BMS, Corrona, LLC, Crescendo, Janssen, Pfizer, Roche/Genentech and UCB,
2,
AbbVie, Amgen, BMS, Corrona, LLC, Crescendo, Janssen, Pfizer, Roche/Genentech and UCB,
9;
G. W. Reed,
Corrona, LLC,
3;
C. Karki,
Corrona, LLC,
3;
R. Magner,
University of Massachusetts Medical School,
3;
J. M. Kremer,
Corrona, LLC,
3,
Corrona, LLC,
1,
Genentech, Inc,
5,
Genentech, Inc,
2;
A. Shewade,
Genentech, Inc,
3;
J. D. Greenberg,
Corrona, LLC,
3,
Corrona, LLC,
1,
AstraZeneca, Celgene, Novartis and Pfizer,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dosing-of-intravenous-tocilizumab-in-a-real-world-setting-analyses-from-a-us-ra-registry/