Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tanezumab is a humanized monoclonal antibody against nerve growth factor that has been evaluated for relief of chronic osteoarthritis pain by subcutaneous (SC) or intravenous (IV) administration every 8 weeks. The current objectives were to (1) characterize the dose-response relationship between assigned dose and selected measures of efficacy at Week 16, (2) determine if average plasma tanezumab concentrations between 12 and 16 weeks (Cav12–16) could explain variability in the exposure-response, and (3) compare dose-response characterization for phase 2 vs phase 3 studies.
Methods: Data were pooled from 7 placebo-controlled clinical studies of tanezumab (2.5, 5, or 10 mg) administered via SC or IV routes in patients with osteoarthritis. Two of the 7 studies also included an active-controlled naproxen arm. Weekly average pain score (WPS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC*) Pain subscale score, and WOMAC Physical Function subscale score were evaluated. Population modeling analyses were conducted, using stepwise covariate modeling and multiple imputation methods consistent with the primary efficacy analysis to account for dropout. Sensitivity analyses used estimates of naproxen vs placebo, with study as an additional covariate on placebo response.
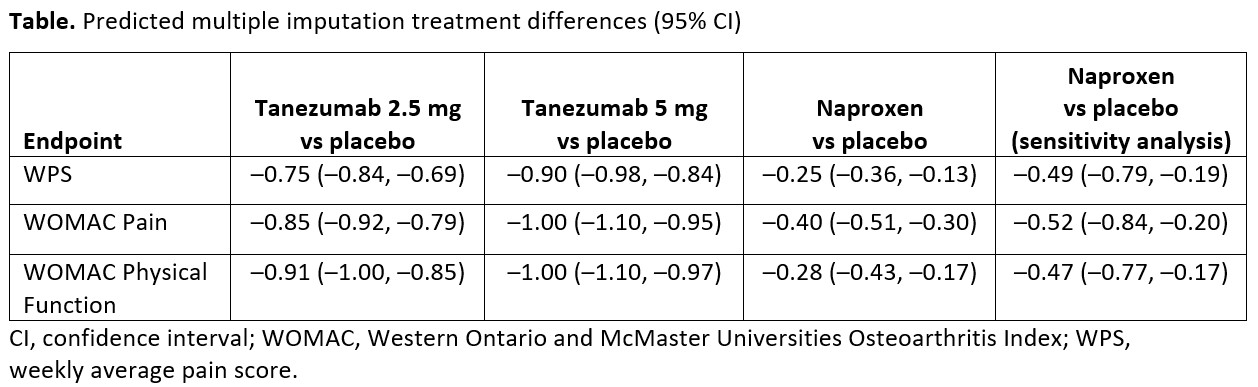
Results: Maximum effect models adequately described the dose-response relationship at Week 16 for all 3 endpoints; Emax point estimates (95% confidence interval [CI]) were: WPS −1.12 (−1.49, −0.76), WOMAC Pain −1.21 (−1.55, −0.87), and WOMAC Physical Function −1.22 (−1.55, −0.90). Model-based estimates of the treatment differences at Week 16 are shown in the Table; a 5 mg dose would provide only a 10–20% increase in effect over a 2.5 mg dose. Sensitivity analysis estimates (95% CI) of treatment differences for naproxen vs placebo for comparison with final model estimates are shown in the
Conclusion: Model-based simulations of Emax showed useful efficacy of pain relief with tanezumab 2.5 mg, with an increase in effect over naproxen. There was little effect gain at a higher tanezumab dose of 5 mg, and comparable efficacy between SC and IV routes of administration.
*© 1996 Nicholas Bellamy. WOMAC® is a registered trademark of Nicholas Bellamy (CDN, EU, USA).
To cite this abstract in AMA style:
Boucher M, Verburg K, Gaitonde P, marshall S. Dose (Exposure) Efficacy Response of Tanezumab Following Intravenous and Subcutaneous Administration Across Phase 2 and Phase 3 Studies in Patients with Osteoarthritis of Hip and Knee [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/dose-exposure-efficacy-response-of-tanezumab-following-intravenous-and-subcutaneous-administration-across-phase-2-and-phase-3-studies-in-patients-with-osteoarthritis-of-hip-and-knee/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dose-exposure-efficacy-response-of-tanezumab-following-intravenous-and-subcutaneous-administration-across-phase-2-and-phase-3-studies-in-patients-with-osteoarthritis-of-hip-and-knee/