Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Observational studies have reported that serum urate positively correlates with bone mineral density (BMD) and that hyperuricaemia is protective for the development of osteoporosis and risk of fragility fractures. In laboratory studies, urate has anabolic effects on bone through differential effects on osteoblast and osteoclast function. However, Mendelian randomization studies do not support a causal role for serum urate in BMD. Inosine is a purine nucleoside that increases serum urate concentrations. The aim of this study was to determine whether moderate hyperuricaemia induced by inosine supplements influences bone turnover markers over a six-month period.
Methods: Six month randomised, double-blind, placebo-controlled trial of 120 post-menopausal female participants. Key exclusion criteria were bone mineral density T-score below -2.5 at the total hip, femoral neck or lumbar spine, previous fragility fracture, bisphosphonate therapy, gout, kidney stones, and urine pH ≤5.0. Participants were randomised 1:1 to either placebo or inosine 500mg twice daily. The co-primary endpoints were change in procollagen type-I N-terminal propeptide (P1NP) and change in β-C-terminal telopeptide of type I collagen (β-CTX). Key secondary endpoints were measures of kidney function, blood pressure and other features of metabolic syndrome. Change in BMD measured by dual-energy x-ray absorptiometry was an exploratory endpoint. Data were analysed on an intention to treat basis, using a mixed models approach to repeated measures.
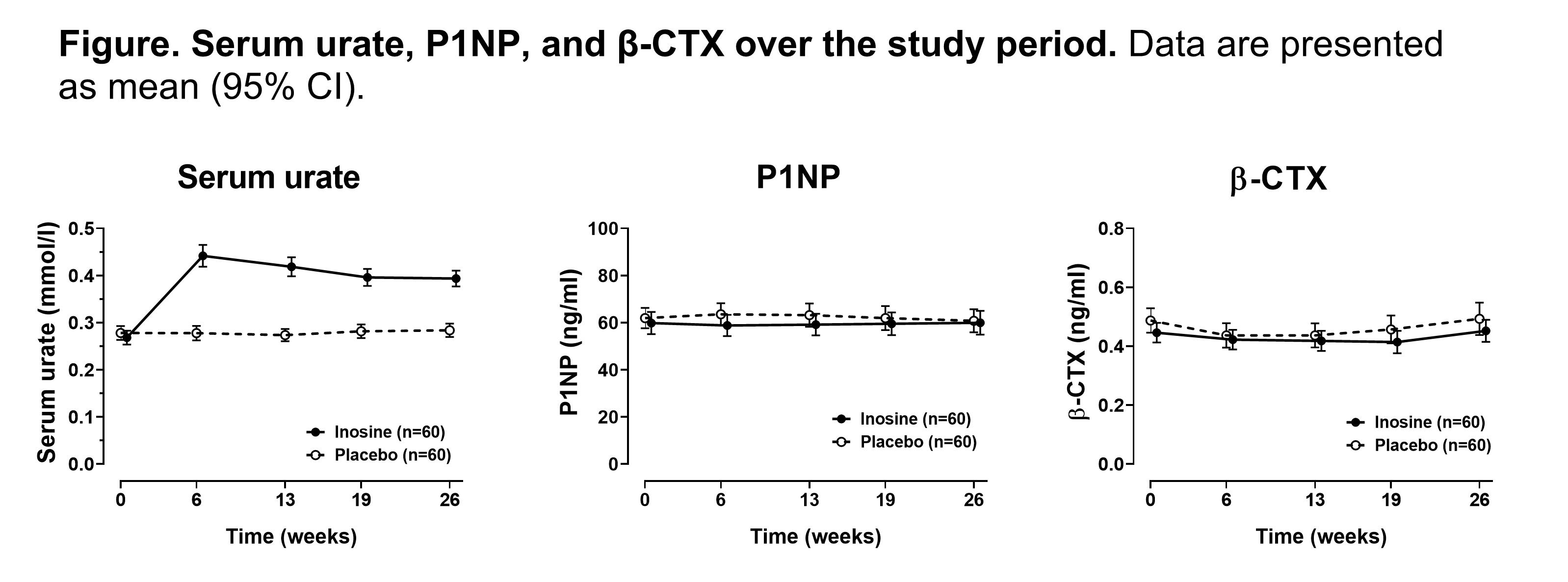
Results: Administration of inosine supplements led to a significant increase in serum urate over the study period (P< 0.0001 for all follow-up time-points, Figure). At week 26, the mean change in serum urate was +0.13 mmol/L (+2.2mg/dL) in the inosine group and 0.00mmol/L (0mg/dL) in the placebo group. There was no difference in P1NP or β-CTX between groups over the six months (false detection rate protected pairwise comparisons at each time point all P > 0.61 for P1NP and P=0.37 for β-CTX, Figure). No significant treatment by time interaction differences were observed in serum creatinine, systolic or diastolic blood pressure, body mass index, fasting lipids, or HbA1c over the study period (all P >0.13). Consistent with the bone turnover marker results, there were no significant changes in BMD between groups over the six months (P >0.48 for all sites). Adverse events and serious adverse events were similar between the two groups.
Conclusion: Although inosine supplementation leads to sustained increases in serum urate over a six month period, it does not alter markers of bone turnover. These findings do not support the concept that urate has direct biological effects on bone turnover.
To cite this abstract in AMA style:
Dalbeth N, Horne A, Mihov B, Merriman T, Gamble G, Stamp L, Reid I. Does Urate Directly Influence Bone Turnover? Randomized Controlled Trial of Inosine Supplementation [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/does-urate-directly-influence-bone-turnover-randomized-controlled-trial-of-inosine-supplementation/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/does-urate-directly-influence-bone-turnover-randomized-controlled-trial-of-inosine-supplementation/