Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is widely used for autoimmune diseases but carries a risk of antimalarial-induced cardiomyopathy (AMIC) that increases with long-term use and is often diagnosed after irreversible heart damage. Endomyocardial biopsy with electron microscopy is the gold standard for diagnosis but is rarely conducted as it is invasive. Screening guidelines, including cardiac biomarkers, ECGs, and imaging, have been proposed to detect early AMIC, but it is not known if they are followed (Tselios et al., 2018; Desmarais et al., 2021; DiGregorio et al., 2025). We aimed to evaluate demographic and clinical features, cardiac biomarkers and cardiac test utilization for patients taking HCQ, according to cumulative HCQ duration, to inform potential risk-based screening approaches.
Methods: We identified patients with ≥10 HCQ prescriptions in the electronic health records (EHR) of our large academic medical center. We extracted patient demographics, rheumatologic diagnoses, HCQ prescription dates, cardiac biomarker testing and results (troponin-I, troponin-T, hsCRP, CK-MB, NT-proBNP), and cardiac testing (ECG, echocardiography [ECHO], cardiac MRI, endomyocardial biopsy). Patients were stratified into 3 groups by HCQ exposure duration: ≤5 years, 5–10 years, and >10 years. Group comparisons were conducted using ANOVA for continuous and Chi-square tests for categorical variables. Cardiac biopsy reports were reviewed to identify AMIC.
Results: Among 13,266 patients (mean age 62.1 [SD 16.9]; 82.9% female), 56.9% had ≤5 years, 22.4% had 5–10 years, and 20.7% had >10 years of HCQ use. While cardiac testing was more common in longer-duration groups, only 70% had ECGs, 41% echocardiograms, and 4.6% cardiac MRI. Importantly, biomarker testing was performed in only a fraction of the total cohort (e.g., troponin-I in 13.3%, CK-MB in 21.6%). Troponin-I abnormalities did increase in prevalence with longer exposure (8.1% in ≤5 years vs 12.4% in >10 years, p=0.02) among those tested. Of 35 patients who underwent biopsy (0.3%), 11 (31.4%) had biopsy-confirmed AMIC.
Conclusion: Despite known risks, cardiac biomarker and imaging tests are underutilized in long-term HCQ users, and biopsy remains rare. Many patients with potential AMIC risk never had testing, limiting recognition of the true disease burden. Our findings highlight a need to develop and implement standardized, non-invasive screening protocols for AMIC to improve early detection and quantify incidence more accurately in high-risk populations.
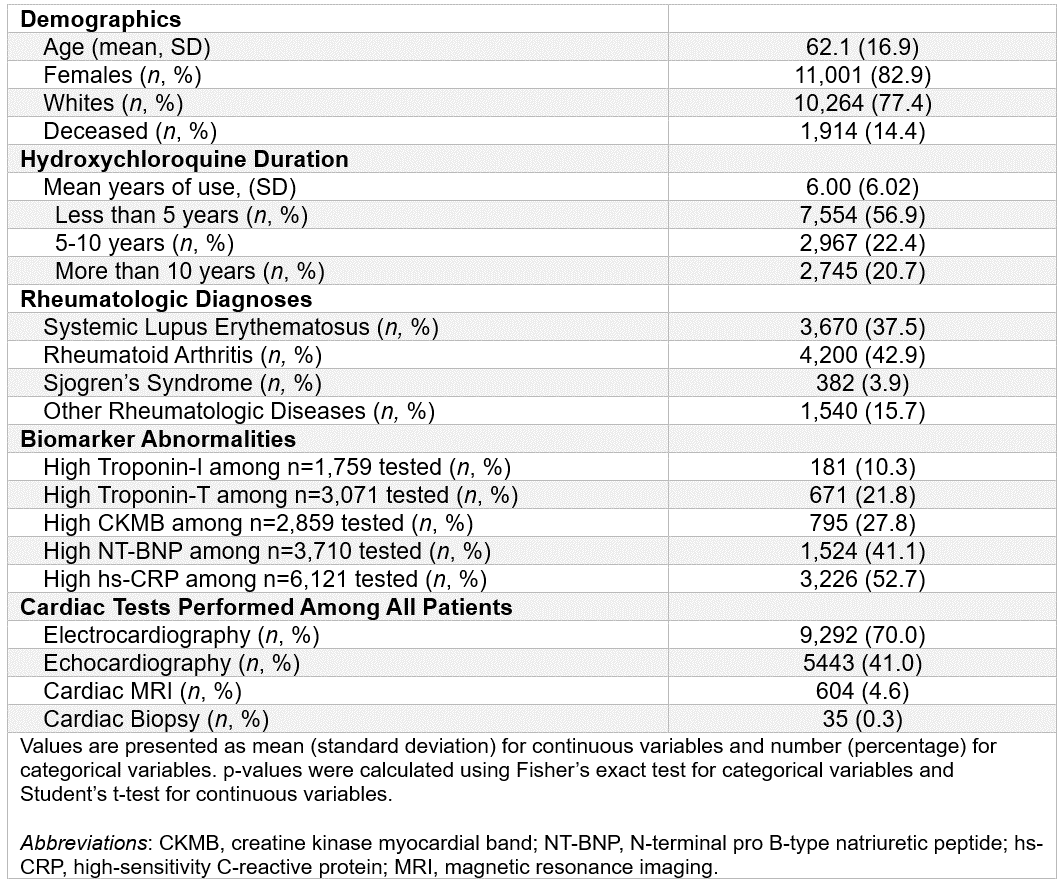 Table 1. Demographics and Clinical Characteristics of Patients with ≥10 Prescriptions of Hydroxychloroquine in a Large Academic Hospital Electronic Health Record Database (n=13,266)
Table 1. Demographics and Clinical Characteristics of Patients with ≥10 Prescriptions of Hydroxychloroquine in a Large Academic Hospital Electronic Health Record Database (n=13,266)
.gif) Table 2. Demographics and Clinical Characteristics of Patients of < 5, 5-10, and >10 years of Hydroxychloroquine Duration in a Large Academic Hospital Electronic Health Record Database (n=13,266)
Table 2. Demographics and Clinical Characteristics of Patients of < 5, 5-10, and >10 years of Hydroxychloroquine Duration in a Large Academic Hospital Electronic Health Record Database (n=13,266)
To cite this abstract in AMA style:
Kim Y, Padera R, Weber B, Costenbader K. Do We Screen For and Do We Miss Antimalarial-Induced Cardiomyopathy (AMIC)? Risk Profiles according to Hydroxychloroquine Exposure Duration [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/do-we-screen-for-and-do-we-miss-antimalarial-induced-cardiomyopathy-amic-risk-profiles-according-to-hydroxychloroquine-exposure-duration/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-we-screen-for-and-do-we-miss-antimalarial-induced-cardiomyopathy-amic-risk-profiles-according-to-hydroxychloroquine-exposure-duration/
