Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Treatment with TNF inhibitors (TNFi) has led to a reduction in signs and symptoms, and improvement in physical function and quality of life in patients with ankylosing spondylitis (AS). Whether TNFi impact the incidence of AS-related comorbidities and extra-articular manifestations (EAMs) is not known.
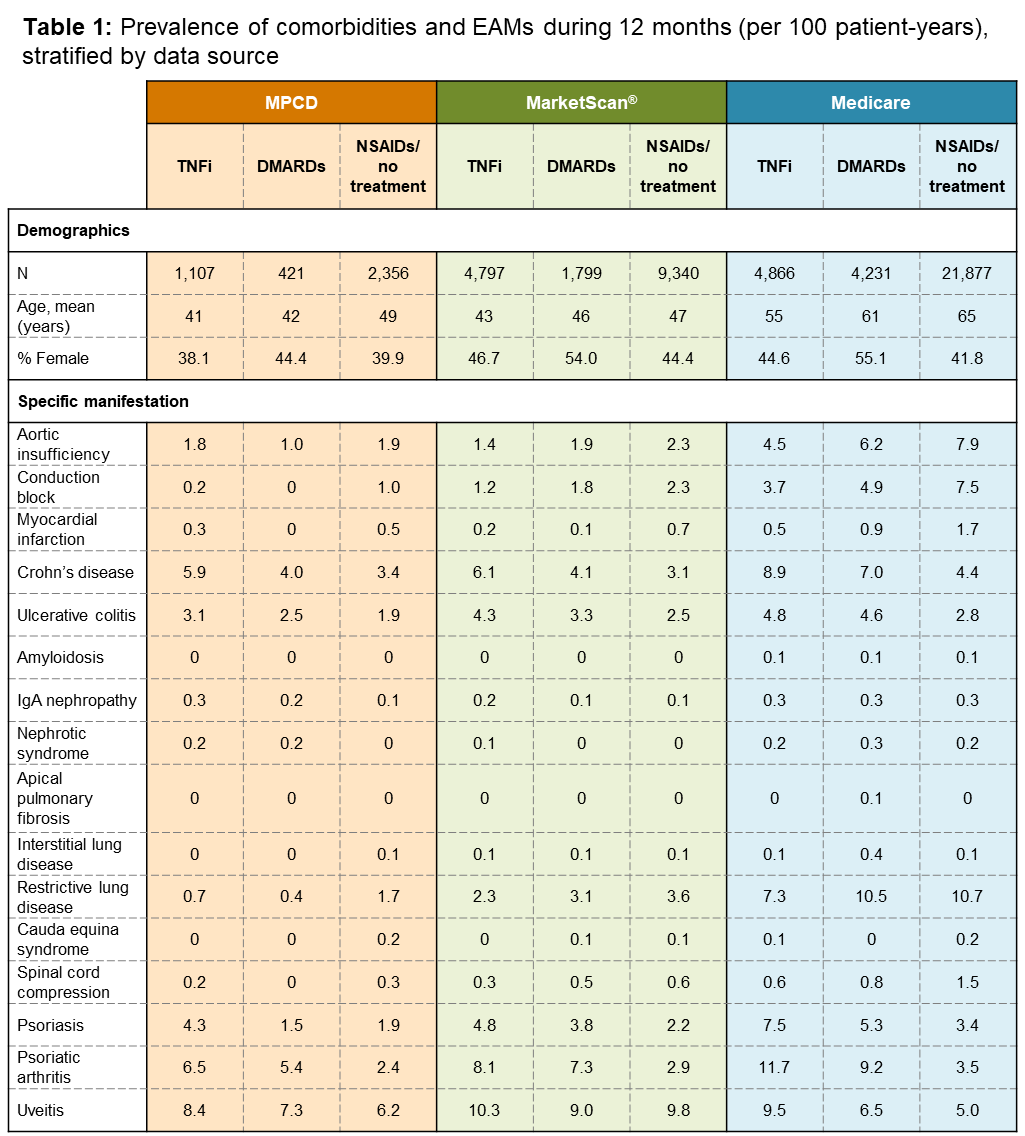
Methods: We conducted a retrospective cohort study using three commercial insurance claims databases (Multi-Payer Claims Database [MPCD; 2007–2010], Truven MarketScan® [2010–2014], and the US Medicare Fee-for-Service Claims data [2006–2014]) to evaluate EAMs (uveitis, psoriasis, inflammatory bowel disease) and comorbidities (cardiac, renal, pulmonary, neurologic) in AS patients diagnosed by a rheumatologist (index date), having 6 months baseline data prior to index date, and drug-specific exposures after AS diagnosis. Three mutually-exclusive hierarchical exposure groups were examined: (1) no therapy or prescription of non-steroidal anti-inflammatory drugs (NSAIDs), (2) conventional disease-modifying antirheumatic drugs (DMARDs), and (3) TNFi. Prevalence of comorbidities were ascertained in 12-month periods (6 months pre- and post-index date). Incidence of comorbidities and EAMs were ascertained during the period following treatment initiation and the earliest of death, loss of medical coverage, end of study, first outcome occurrence, treatment discontinuation, or initiation of therapy at a higher level in exposure hierarchy. Comparisons were made using the mid-p exact test (α=0.05).
Results: Out of nearly 40 million beneficiaries, 63,052 patients were included. Table 1 shows the prevalence of comorbidities and EAMs of AS by treatment exposures, stratified by data source. Comorbidities were more common in Medicare AS patients compared to MPCD or MarketScan®. Table 2 shows the incidence rates of outcomes by treatment exposures, stratified by data source. Despite the possibility of patients with more severe disease receiving TNFi treatment, their crude incidence of certain cardiac, pulmonary and neurologic comorbidities was lower compared to those treated with NSAIDs or DMARDs alone, although they had higher incidence of some EAMs.
Conclusion: This was the largest investigation of the prevalence and incidence of comorbidities and EAMs of AS within the US, and suggests TNFi to be disease-modifying. In the absence of control for confounding, these findings should be considered preliminary.
To cite this abstract in AMA style:
Deodhar AA, Winthrop K, Chan B, Siegel SAR, Pisenti L, Stark J, Suruki RY, Bohn RL, Yun H, Chen L, Curtis JR. Do TNF Inhibitors Alter the Natural History of Ankylosing Spondylitis By Impacting the Incidence and Prevalence of Comorbidities and Extra-Articular Manifestations? [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/do-tnf-inhibitors-alter-the-natural-history-of-ankylosing-spondylitis-by-impacting-the-incidence-and-prevalence-of-comorbidities-and-extra-articular-manifestations/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-tnf-inhibitors-alter-the-natural-history-of-ankylosing-spondylitis-by-impacting-the-incidence-and-prevalence-of-comorbidities-and-extra-articular-manifestations/