Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Conventional synthetic DMARDs remained the mainstay of treatment of RA for decades. However, their use has decreased recently due to the emergence of biological and targeted synthetic DMARDs like JAK Inhibitors. Regrettably, the high cost of biologics has limited access for many patients in economically disadvantaged regions. Therefore, in most developing nations like India, the preferred initial treatment for RA is a combination of cs-DMARDs
Methods: This PRospective Observational Study to Estimate the Extent of Response to Conventional Synthetic DMARDs in RA (PROSEED-RA) study enrolled consecutive RA patients with a disease duration of less than 3 years, excluding those previously treated with biologicals or ts-DMARDs. Approval for the study was secured from the Institutional Review Board (IRB) at the esteemed Christian Medical College, Vellore, Tamil Nadu, India. Baseline data encompassed age, disease duration, comorbidities, socioeconomic status, smoking history, steroid usage, type and dosage of DMARD regimen (monotherapy, dual therapy, or triple therapy), and disease activity metrics (CDAI, SDAI, and DAS28), recorded initially and at 3-month intervals over one year. The primary endpoint is the proportion of patients achieving remission or low disease activity at the end of 1 year period. Treatment strategies—like monotherapy, dual therapy, or triple therapy – were initiated at the discretion of attending physicians. Patients lost to follow-up or non-compliant with treatment were excluded from the final analysis. Adverse events were meticulously documented, with the transition from csDMARDs to biological or tsDMARDs indicating treatment failure
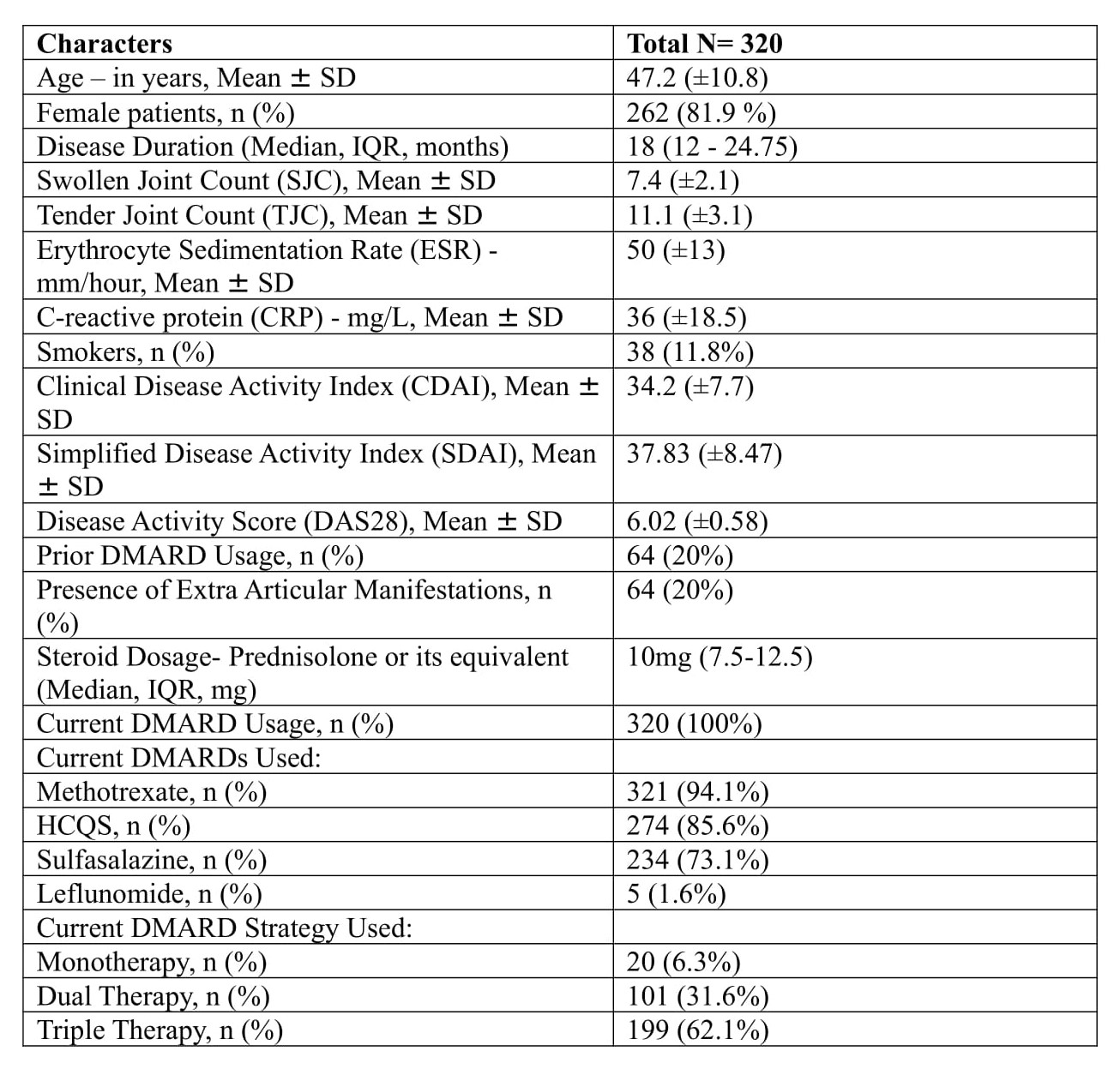
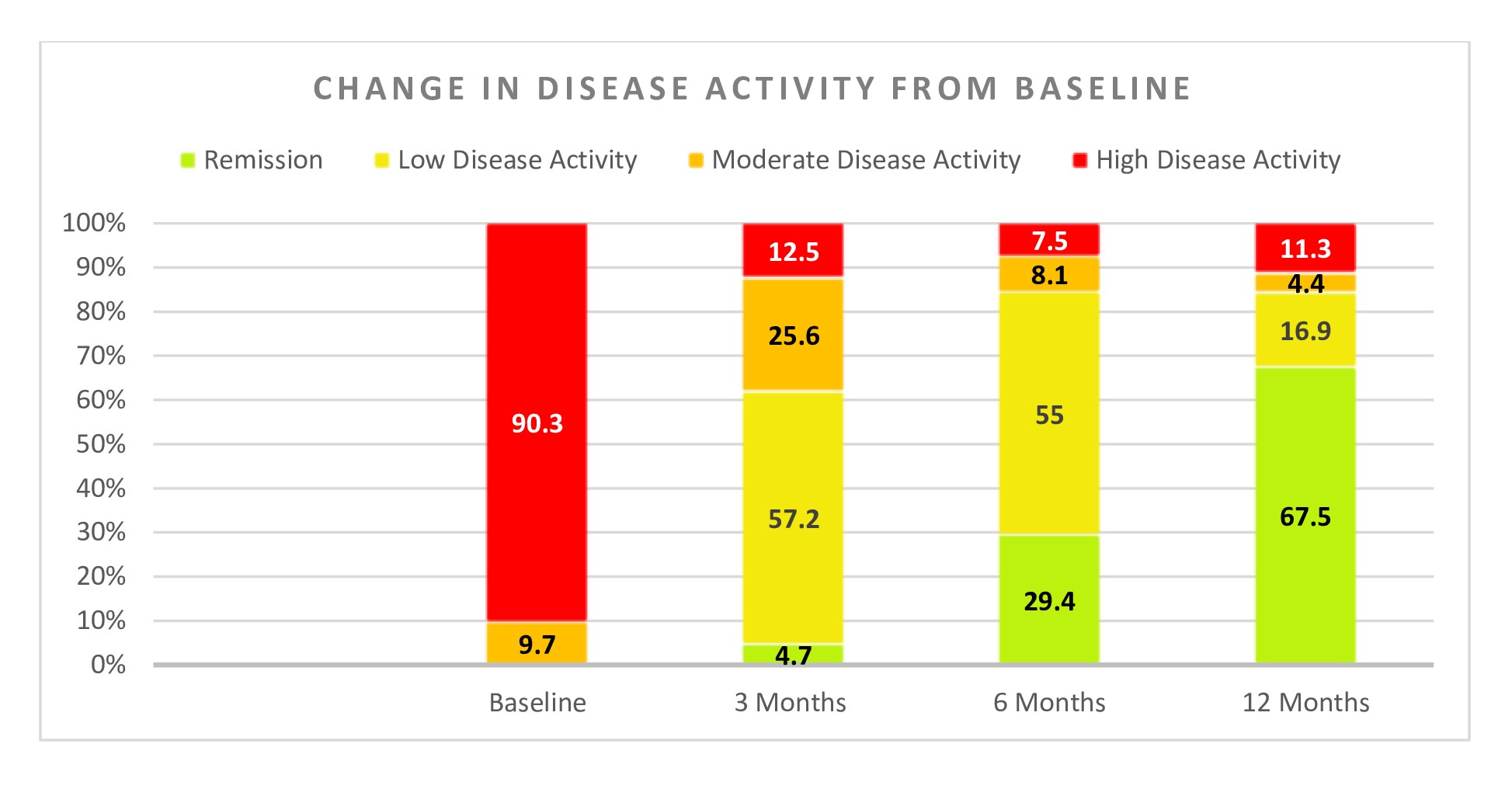
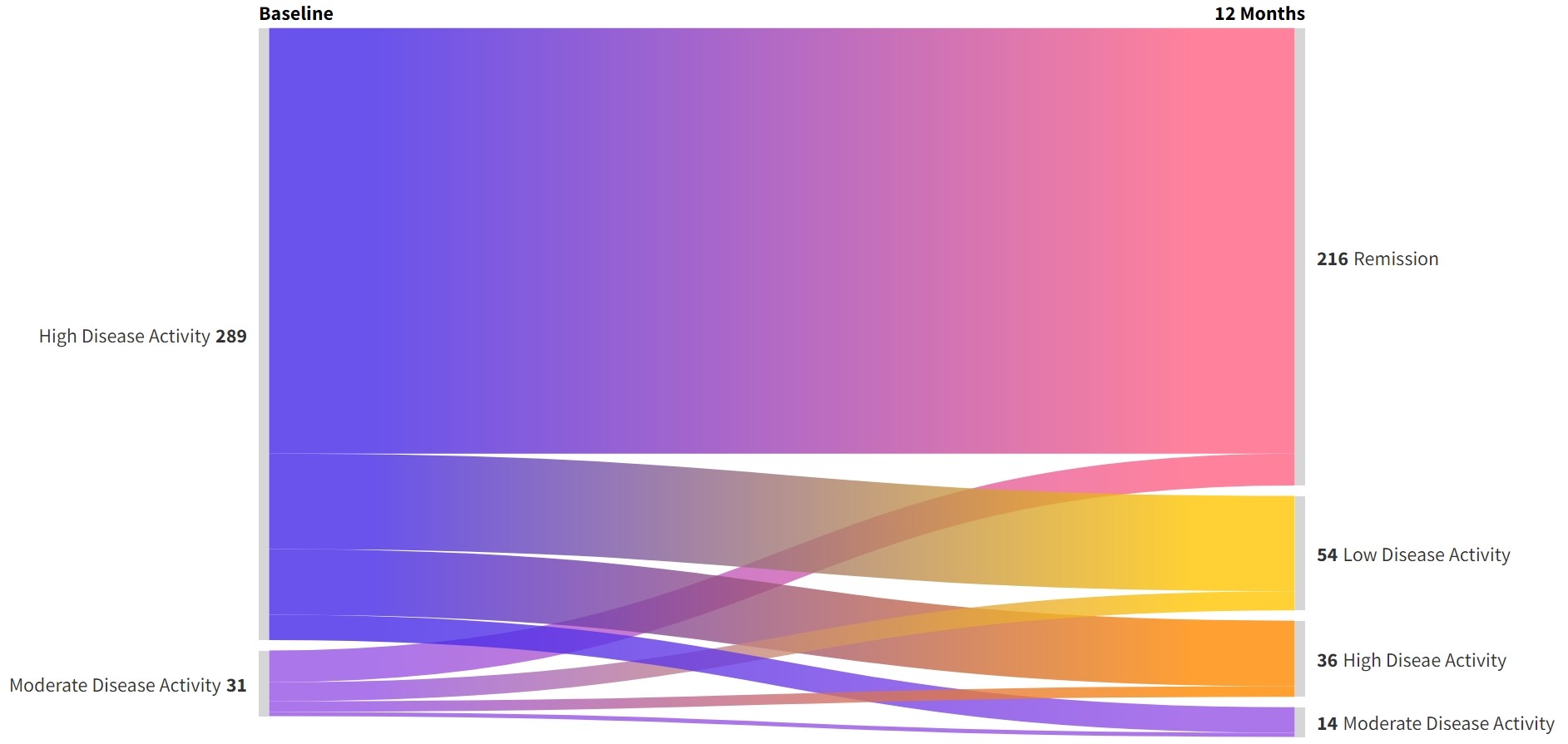
Results: 350 patients were initially enrolled, but 30 individuals were later excluded due to drug default or missed follow-up. Ultimately, data from 320 patients with a median disease duration of 18 months were included in the final analysis. Their baseline data is represented in Table 1. At the end of the one-year study period, 270 out of 320 patients (84.4%) achieved either low disease activity (LDA) or complete remission (Figure 1&2). Specifically, 216 patients (67.5%) achieved complete remission, while 54 (16.9%) attained LDA. Among those who achieved remission or LDA, 20 patients (7.5%) were on monotherapy, 95 (35%) were on dual therapy, and 155 (57.5%) were on triple therapy. The median steroid dosage decreased to 1.25mg (0-2.5mg) at the end of the study.
Factors predicting non-response included male sex, smoking history, and prior use of DMARDs. Other parameters such as a higher baseline SJC/TJC, CDAI/SDAI/DAS28 scores did not significantly influence outcomes in non-responders, nor did the presence of comorbidities, extra-articular manifestations, or socioeconomic status. 32 patients (10%) discontinued csDMARDs due to lack of efficacy, categorized as treatment failures. No significant adverse events were reported throughout the study period
Conclusion: The use of combination csDMARDs as the primary treatment for RA in Indian patients remains efficacious, even in the current era of JAKIs and Biologicals. This reaffirms the adage “Old is Gold” underscoring the effectiveness of csDMARDs and their use should be encouraged in clinical practice
To cite this abstract in AMA style:
Vasanth P, Mathew J, K D. Do CsDMARDs Still Play a Significant Role in Managing Rheumatoid Arthritis in the Current Biologic/JAKI Era? A Prospective Observational Study to Estimate the Extent of Response to Conventional Synthetic DMARDs in RA– Experience from a Tertiary Care Center in South India [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/do-csdmards-still-play-a-significant-role-in-managing-rheumatoid-arthritis-in-the-current-biologic-jaki-era-a-prospective-observational-study-to-estimate-the-extent-of-response-to-conventional-synthe/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-csdmards-still-play-a-significant-role-in-managing-rheumatoid-arthritis-in-the-current-biologic-jaki-era-a-prospective-observational-study-to-estimate-the-extent-of-response-to-conventional-synthe/