Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: FINCH 1 (NCT02889796) was a Phase 3 randomized controlled trial evaluating filgotinib (FIL) in patients with rheumatoid arthritis and an inadequate response to methotrexate (MTX).1 Patients received background MTX and were randomized 3:3:2:3 to FIL 200 mg (FIL200), FIL 100 mg, adalimumab or placebo. This analysis aimed to identify patterns of response trajectory over 12 months in patients from FINCH 1 receiving FIL200.
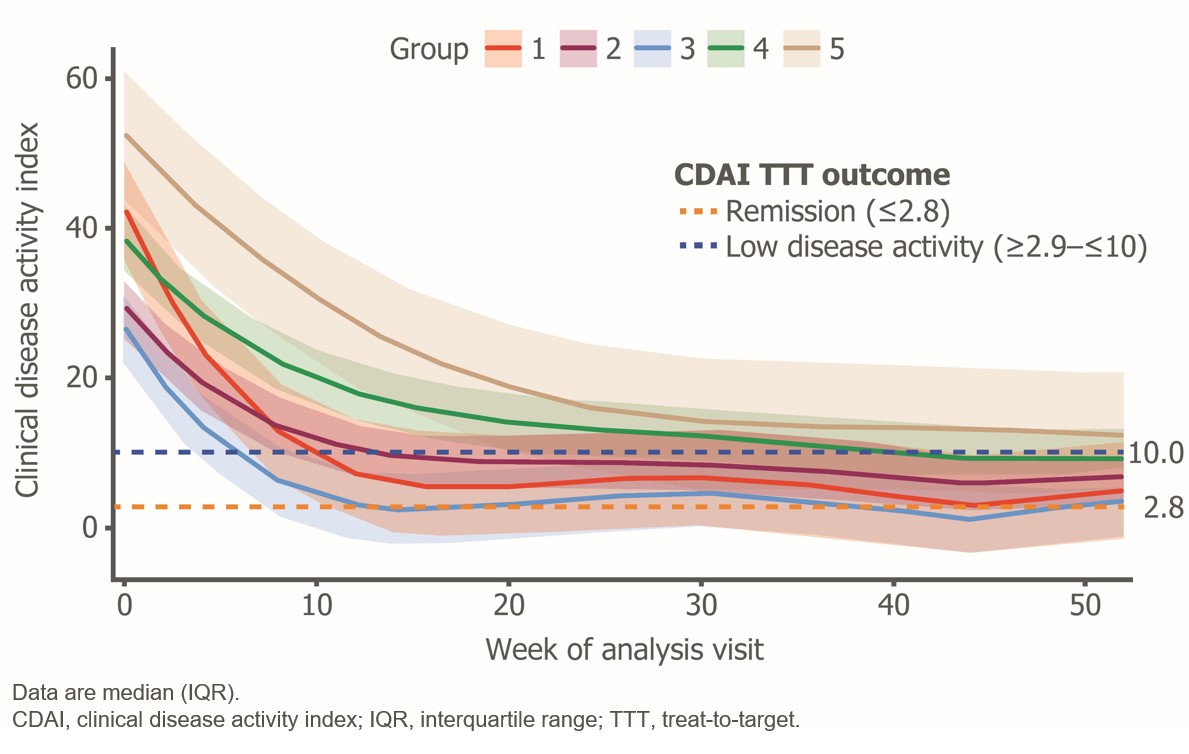
Methods: Group-based trajectory modeling is a statistical method which groups individuals based on similar patterns of change in an outcome over time.2 A group-based trajectory modeling approach was applied to identify five distinct phenotypic groups in terms of observed clinical disease activity index (CDAI) outcomes (and components) over a 12-month period (Figure). Responders were recorded as either low disease activity (LDA) or remission, defined as CDAI between ≥ 2.9 and ≤ 10, or ≤ 2.8, respectively, at 6 months.
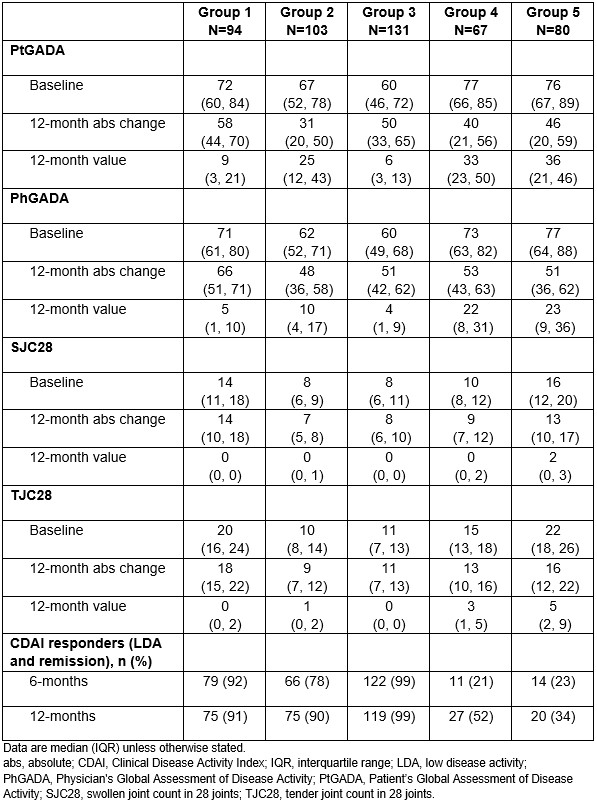
Results: Mean age, the proportion of females and disease duration were similar across all groups. Improvements in efficacy outcome measures were seen for all groups. Baseline (median interquartile range [IQR]) CDAI was highest (worst) in Group 5 (52 [48, 60]), followed by Group 1 (48 [44, 55]). Groups 1 and 3 showed a rapid reduction in CDAI in the first 6 months and sustained response over 12 months. Groups 2, 4 and 5 demonstrated slower response trajectories, with continued improvements in the proportion of CDAI responders between 6 and 12 months; of note, Group 5 constituted 16.8% (80/475) of the total analysis population. Table shows that despite substantial and comparable improvements in CDAI components across all 5 groups over 12 months, Group 5 demonstrated some residual disease activity.
Conclusion: A group-based multivariate trajectory model identified five distinct CDAI disease activity trajectories in patients from FINCH 1 receiving FIL200. Patients in all groups demonstrated either fast and sustained response or slower and continued improvements, with no or low numbers of swollen or tender joints at Month 12. The current analysis suggests that patients who do not achieve CDAI LDA within 6-months may still benefit from treatment continuation and informs a range of expectations for times to clinical response. Future work will assess biomarker profiles that may be used to predict the observed clinical response patterns.
References:
1. Combe B, et al. Ann Rheum Dis 2021;80:848–58
2. Nagin DS, et al. Stat Methods Med Res 2018;27:2015–23
To cite this abstract in AMA style:
Taylor P, Tanaka Y, Aiello E, Debray T, Watson C, Harris K, Burmester G. Distinct Treatment Responses in Patients with Rheumatoid Arthritis Receiving Filgotinib 200 Mg over 12 Months: A Post Hoc Analysis of FINCH 1 [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/distinct-treatment-responses-in-patients-with-rheumatoid-arthritis-receiving-filgotinib-200-mg-over-12-months-a-post-hoc-analysis-of-finch-1/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/distinct-treatment-responses-in-patients-with-rheumatoid-arthritis-receiving-filgotinib-200-mg-over-12-months-a-post-hoc-analysis-of-finch-1/