Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is a serious extra-articular manifestation of RA, affecting 7-12% of patients and associated with three-fold increased mortality compared to RA without ILD. The associations between the use of DMARDs and the development of ILD among patients with RA remain unclear. Thus, we conducted a systematic literature review for studies investigating DMARDs for incident ILD risk among patients with RA.
Methods: This systematic literature review and meta-analysis was registered with PROSPERO (CRD42023485052). PubMed, Embase, Web of Science, and Cochrane Library were searched from inception to November 2023 for randomized controlled trials (RCTs), observational studies, and post-marketing surveillance studies that examined the associations of DMARDs and risk of incident RA-ILD. The outcome was incident ILD. We assessed for risk of bias using Cochrane risk of bias tool 2.0 for RCTs and Newcastle-Ottawa scales for observational studies. Among studies with sufficient quality, we performed meta-analyses to obtain odds ratios (OR) and 95% confidence intervals (CI) using the Mantel-Haenszel methods. Heterogeneity was evaluated using Q-test and quantified with the I2 statistic, with high heterogeneity defined as p<0.1 for the Q-test or I2>50%.
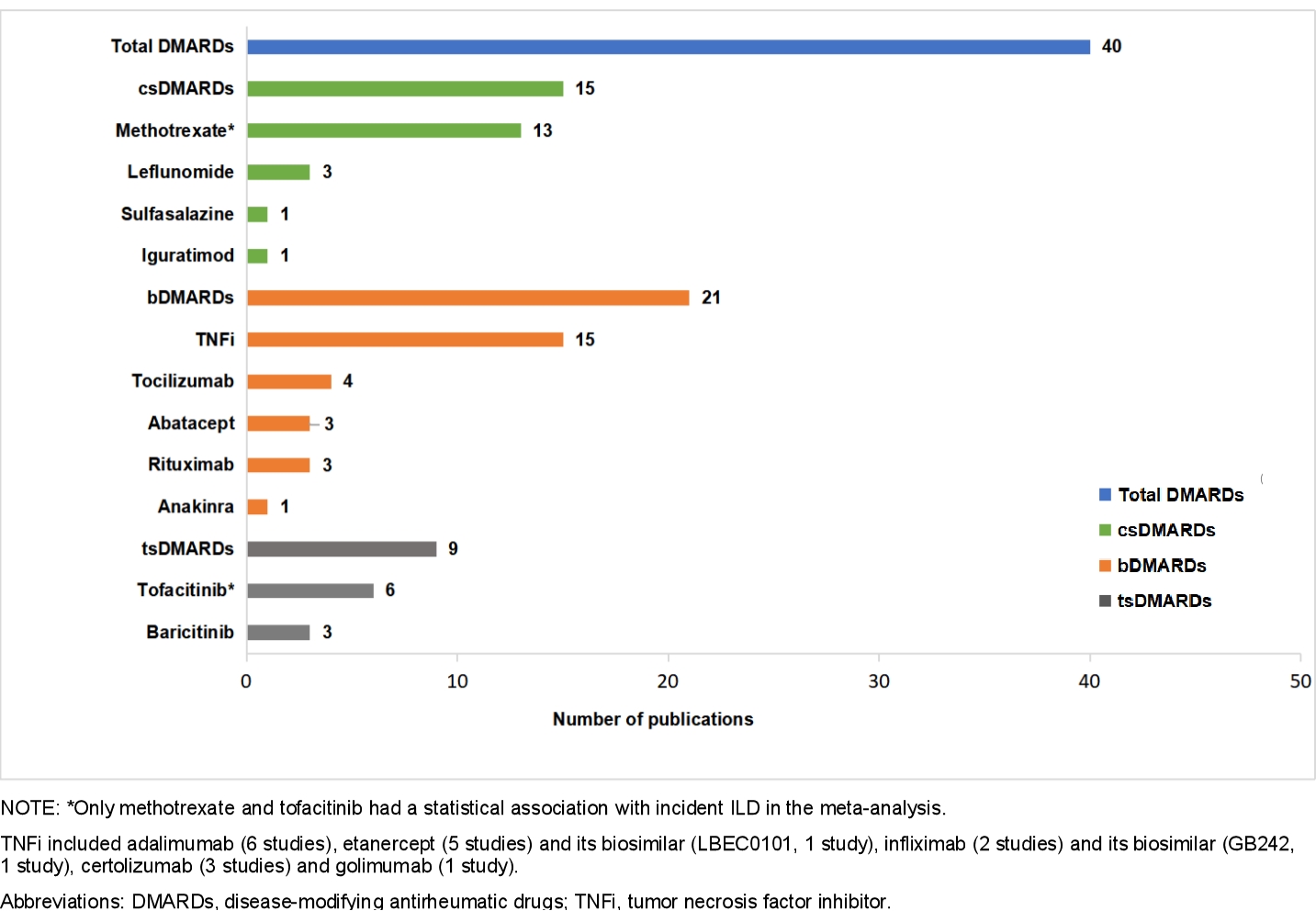
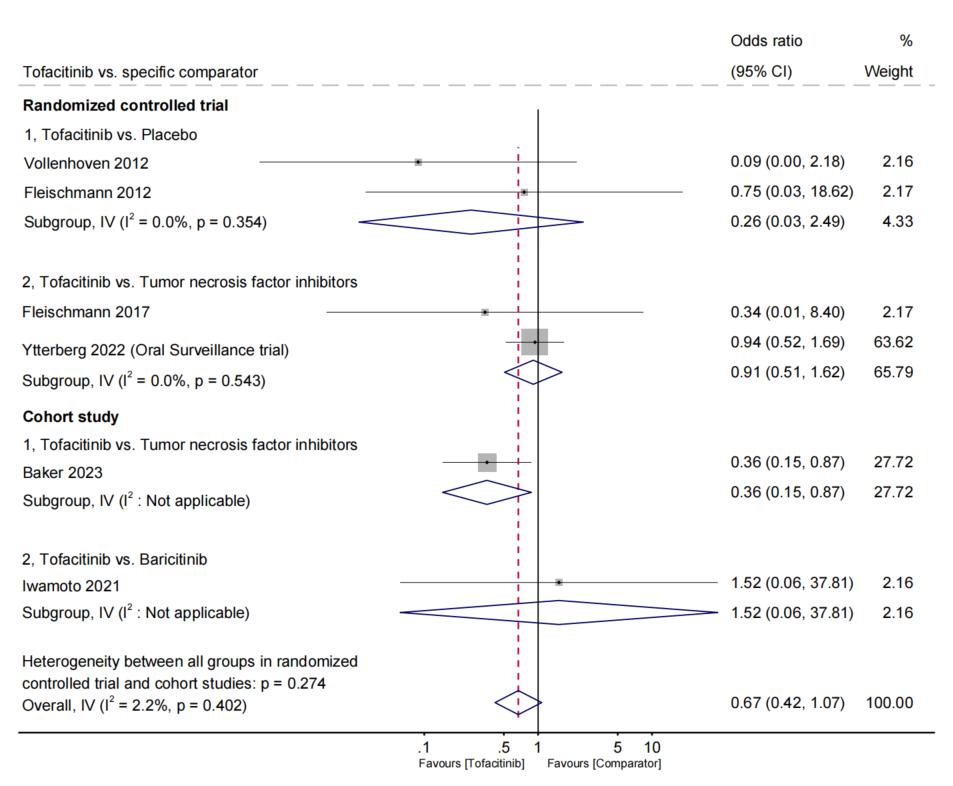
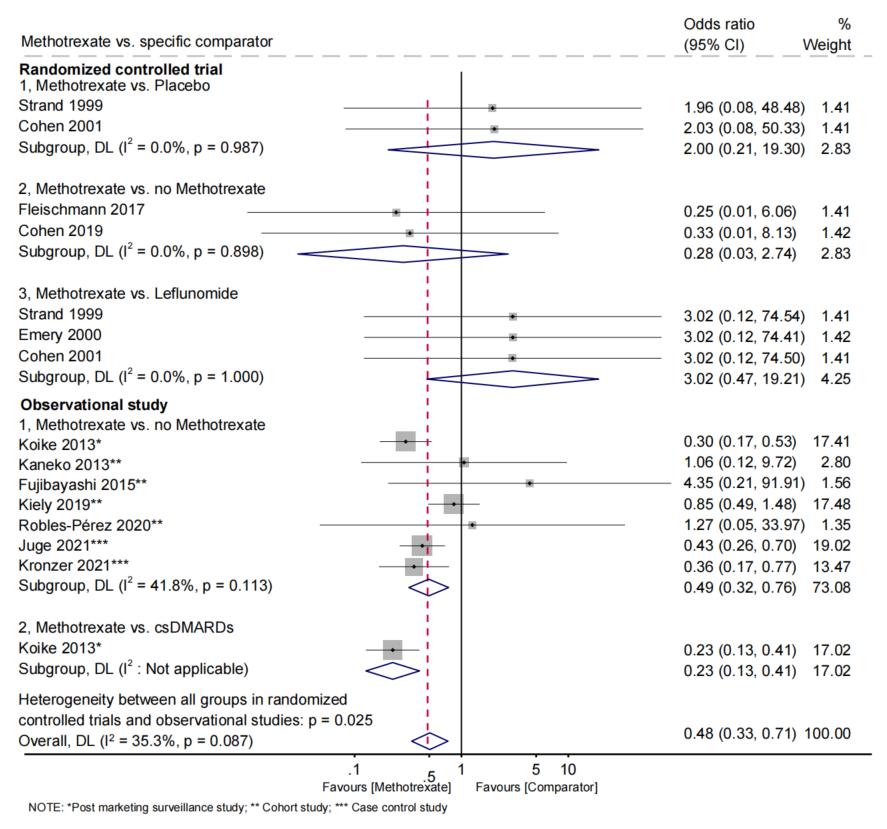
Results: Among 3,612 studies, we identified a total of 40 eligible studies with sufficient quality, encompassing 486,465 adult patients with RA and 3,928 incident ILD. We analyzed 24 RCTs, 4 prospective cohort studies, 9 retrospective cohort studies, 2 case-control studies, and 1 post-marketing surveillance study. There were 15 studies focused on csDMARDs (most frequently methotrexate [MTX]), 21 on bDMARDs (most frequently tumor necrosis factor inhibitors [TNFi], and 9 on tsDMARDs (most frequently tofacitinib; Figure 1). The pooled analyses from RCTs revealed no statistically significant difference in the odds of ILD development for any specific DMARD across all comparisons examined. The largest identified RCT (Oral Surveillance trial) of tofacitinib (n=2,911) vs. TNFi (n=1,451) found no relationship with incident ILD (OR 0.94, 95%CI 0.52 to 1.69, p=0.83; Figure 2). In 7 observational studies, MTX users had a pooled OR for ILD of 0.49 (95%CI 0.32 to 0.76, p< 0.001; I2 and Q-test, I2=41.8%, p=0.113; Figure 3) compared to those not using MTX. In a single observational study, tofacitinib users had an OR for ILD of 0.36 (95%CI 0.15 to 0.87, p=0.024) compared to TNFi users.
Conclusion: Observational data suggested a potential protective role of MTX and tofacitinib for incident RA-ILD risk. However, these studies may be susceptible to confounding by indications, and no specific DMARD showed associations with incident RA-ILD in RCTs. Further well-designed prospective trials are warranted for definitive conclusions on the relationship between DMARDs and RA-ILD risk.
To cite this abstract in AMA style:
Zhang Q, McDermott G, Chang S, Juge P, Vanni K, Qian G, Bade K, Mueller K, Kowalski E, Saavedra A, Sparks J. Disease-modifying Antirheumatic Drugs and Risk of Incident Interstitial Lung Disease Among Patients with Rheumatoid Arthritis: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/disease-modifying-antirheumatic-drugs-and-risk-of-incident-interstitial-lung-disease-among-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-modifying-antirheumatic-drugs-and-risk-of-incident-interstitial-lung-disease-among-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/