Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Rheumatoid arthritis (RA) is an chronic inflammatory disease that is frequently treated with tumor necrosis factor inhibitors (TNFi). Little is known about predictors of response to TNFi. As clinical response in RA is measured by composite scores that contain a substantial number of subjective patients-orientated domains (pain, global disease perception) we speculated that patients with high disease representation in the central nervous system (CNS) may respond better to TNFi than patients in whom the disease is less represented in the CNS.
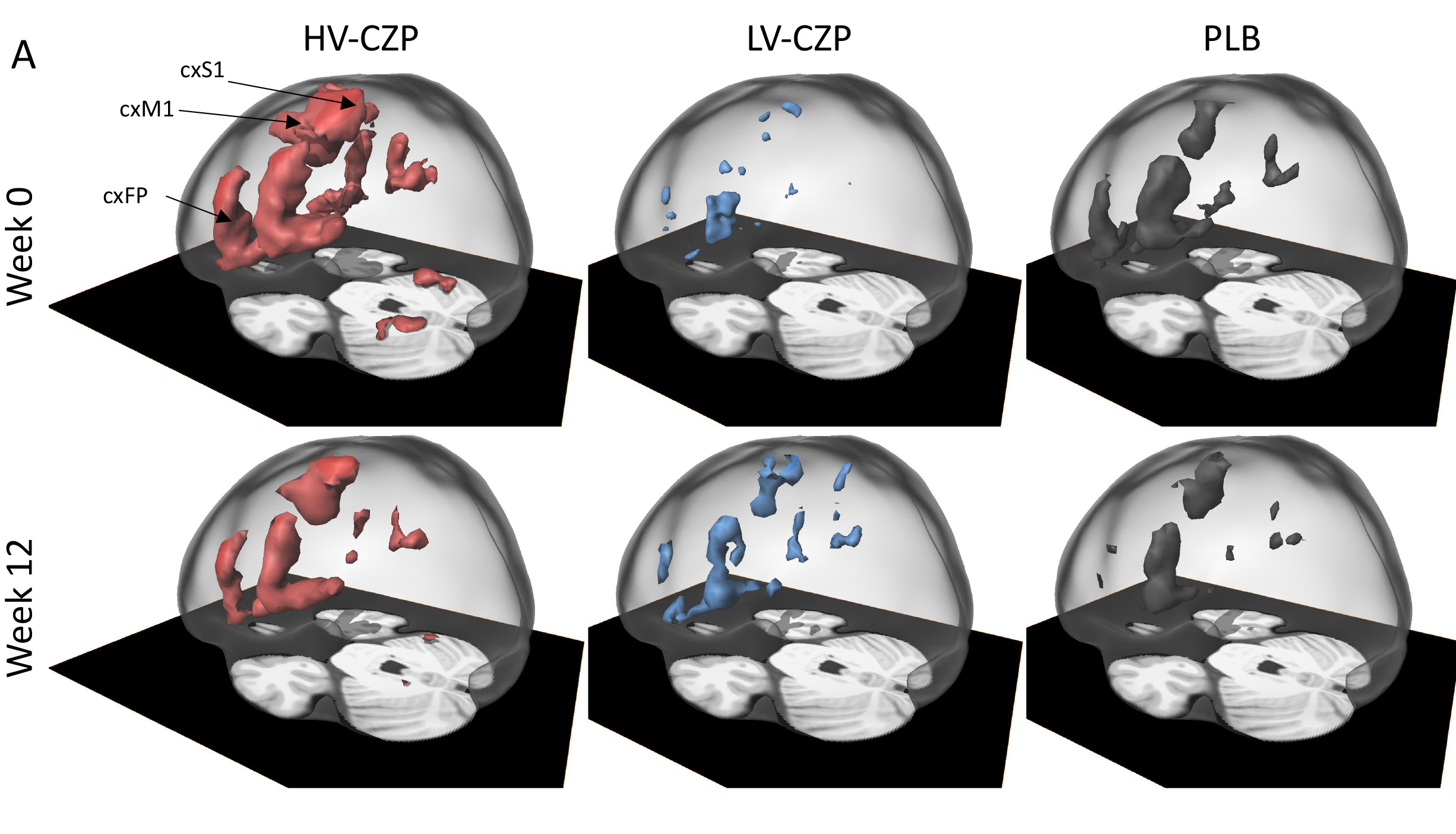
Methods: Phase 3,international multicenter, double-blind, placebo-controlled, parallel-group study with active RA patients. All patients underwent a functional MRI (fMRI) scan of the CNS at baseline measuring central nervous system CNS activation (voxels) by joint compression. Patients were stratified according to fMRI (high voxel, HV; low voxel, LV) and randomized 2:1 into treatment with the TNFi certolizumab-pegol (CZP) or placebo (PLB). The primary efficacy analysis compared the percentages of patients reaching low disease activity (LDA) according to DAS28 (≤3.2) at week 12. In addition, exploratory multi-parametric MRI assessment of brain anatomy and function was performed and analyzed with machine-learning approaches.
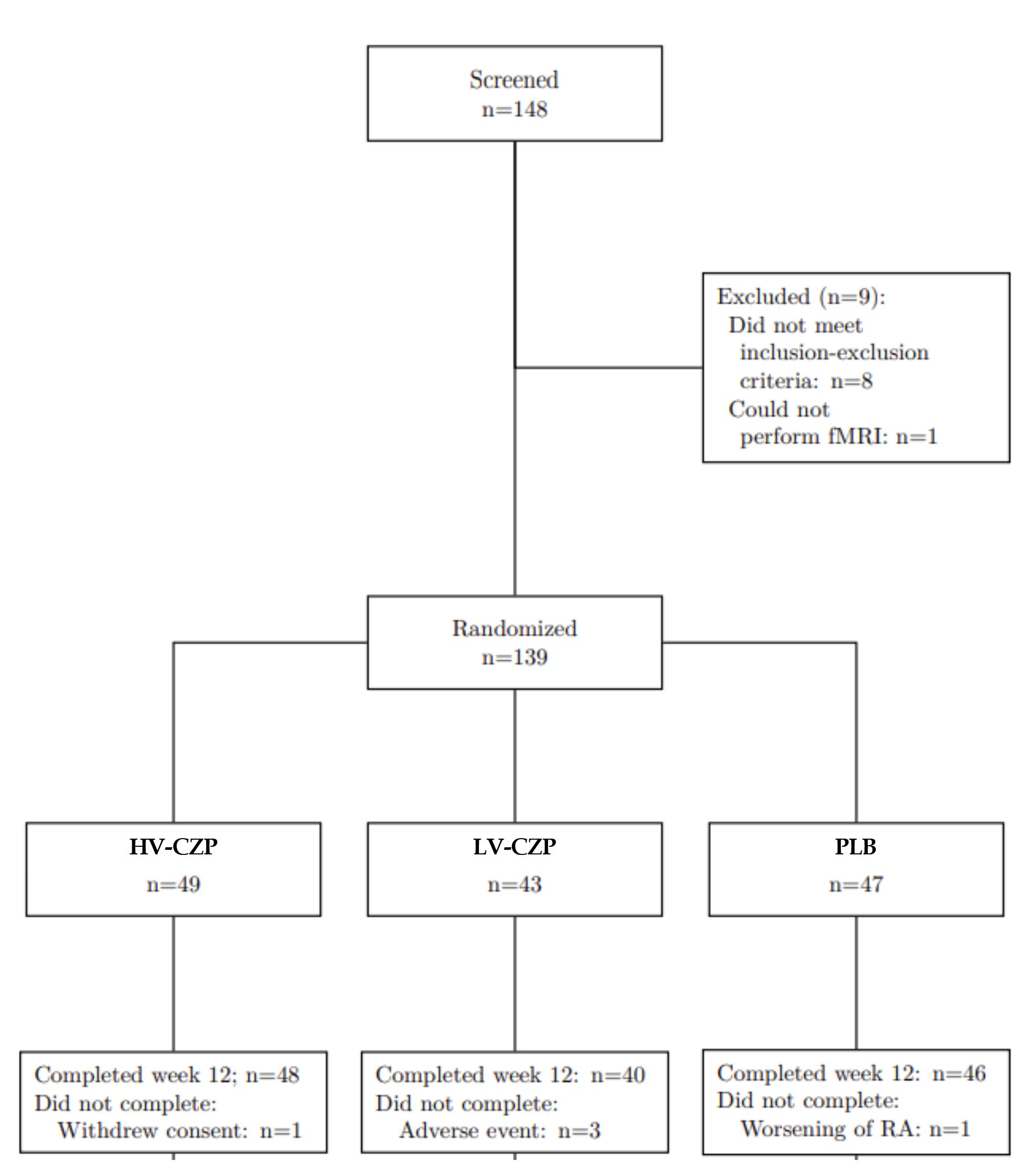
Results: 148 RA patients were screened and 139 patients were randomized to the HV-CZP arm(N=49), the LV-CZP arm (N=43) or PLB arm(N=47)patients. LDA was achieved by28/49 (57%) in HV-CZP,19/43 (44%) in LV-CZP and12/47 (26%) in PLB group at week 12. Response in the HV-CZP group but not in the LV-CZP group were significantly (p< 0.002) different from PLB group. HV-CZP and LV-CZP responses differed from placebo in patient-oriented outcomes (pain, patients global) but were similar in objective measures (CRP, joint swelling).The multi-parametric MRI together with machine learning confirmed, that only fMRI voxel size was able to separate CZP and PLB with an accuracy of 95.2%
Conclusion: High disease-associated CNS activation,measured by fMRI,predicts good clinical response of RA patients to TNFi.
criteria and 1 patient was not able to perform fMRI. Finally 139 patients were randomized into 3 groups:
92 patients received Certolizumab-Pegol (CZP), and were allocated to 2 different groups, stratified by the
fMRI results at baseline: 49 patients were included in the high voxel count (HV-CZP)- group and 43 patients
were included in the low voxel count (LV-CZP)- group. 47 patients were assigned to the placebo control
(PLB) group.
To cite this abstract in AMA style:
Rech J, Hess P, Tascilar K, Schenker D, schönau v, Sergeeva M, Prade J, Kreitz S, Sulvakumar M, Konerth L, Strobelt S, Englbrecht M, Hueber P, Feist E, Zaiss P, Burmester G, Behrens F, Koehm D, Baerwald P, Finzel D, Kleyer D, Voll P, Roesch D, Doerfler P, Damjanov P, Da Silva P, Schett G. Disease-associated Central Nervous System Activation Predicts Good Clinical Response to Tumor Necrosis Factor Inhibition in Rheumatoid Arthritis Patients – “The PreCePRA Study” [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/disease-associated-central-nervous-system-activation-predicts-good-clinical-response-to-tumor-necrosis-factor-inhibition-in-rheumatoid-arthritis-patients-the-precepra-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-associated-central-nervous-system-activation-predicts-good-clinical-response-to-tumor-necrosis-factor-inhibition-in-rheumatoid-arthritis-patients-the-precepra-study/