Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Tofacitinib (TOFA) is an oral, small molecule drug used for rheumatoid arthritis (RA) treatment as the first or an alternative option to biologic disease- modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor inhibitors (TNFi). The similarity in retention of TNFi and TOFA was previously reported separately by the Ontario Best Practices Research Initiative (OBRI) and the Quebec cohort RHUMADATA. To increase the study power, we propose to evaluate the discontinuation rate (due to any reason) of TNFi compared to TOFA, using pooled data from both these registries.
Methods: RA patients enrolled in the OBRI and RHUMADATA initiating their TOFA or TNFi between 1st June 2014 (TOFA approval date in Canada) and 31st Dec 2019 were included. Time to discontinuation was assessed using adjusted Kaplan-Meier (KM) survival and Cox regression models. To deal with confounding by indication, we estimated propensity scores for covariates with a standard difference greater than 0.1. Models were then adjusted using stratification and inverse probability of treatment weight (IPTW) methods. Multiple imputation (Imputation by Chained Equation method, N=20) was used to deal with missing data for covariates at treatment initiation.
Results: A total of 1318 patients initiated TNFi (n=825) or TOFA (n=493) with mean (SD) disease duration of 8.9 (9.3) and 13.0 (10.1) years, respectively. In the TNFi group, 78.8% were female and mean age (SD) at treatment initiation was 57.6 (12.6) years. In the TOFA group, 84.6% were female and mean (SD) age at treatment initiation was 59.5 (11.5) years. The TNFi group was less likely to have prior biologic use (33.9%) than the TOFA group (66.9%). At treatment initiation, the mean (SD) CDAI was significantly (p< 0.05) lower in the TNFi group [20.0 (11.7)] compared to the TOFA group [22.1(12.4)]. Physical function measured by HAQ-DI was also significantly lower (p< 0.05) in the TNFi compared to the TOFA group (1.2 vs.1.3).
Over a mean follow-up of 23.2 months, discontinuation was reported in 309 (37.5%) and 182 (36.9%) of all TNFi and TOFA patients, respectively. After adjusting for propensity score deciles across 20 imputed datasets, there was no significant difference in discontinuation between treatment groups (adjusted HRs: 0.96, 95% CI: 0.78-1.18; p=0.69). The results were similar for two propensity adjustment methods. Figure 1 shows IPTW adjusted KM survival curves comparing discontinuation rates in patients treated with TNFi and TOFA.
Conclusion: In this pooled real -world data study, we found that TNFi and TOFA retention is similar in patients with RA. In the next step we will analysis the data for specific reasons of discontinuation. We will also repeat analysis comparing discontinuation in the first users versus those after one or more biologic failure.
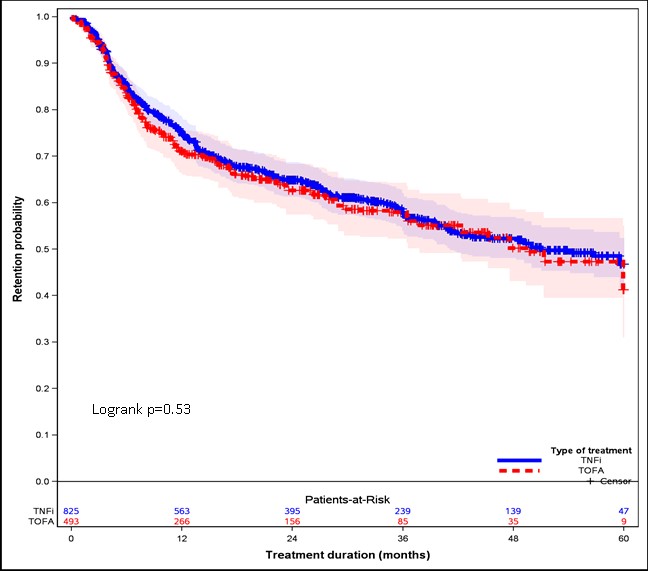 Figure 1. Note: Propensity Score Weighted (IPTW) Survival Curves was performed using one imputed dataset
Figure 1. Note: Propensity Score Weighted (IPTW) Survival Curves was performed using one imputed dataset
To cite this abstract in AMA style:
Movahedi M, Choquette D, Coupal L, Cesta A, Li X, Keystone E, Bombardier C. Discontinuation Rate of Tofacitinib Is Similar When Compared to TNF Inhibitors in Rheumatoid Arthritis Patients: Pooled Data from Two Rheumatoid Arthritis Registries in Canada [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/discontinuation-rate-of-tofacitinib-is-similar-when-compared-to-tnf-inhibitors-in-rheumatoid-arthritis-patients-pooled-data-from-two-rheumatoid-arthritis-registries-in-canada/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-rate-of-tofacitinib-is-similar-when-compared-to-tnf-inhibitors-in-rheumatoid-arthritis-patients-pooled-data-from-two-rheumatoid-arthritis-registries-in-canada/
