Session Information
Date: Tuesday, October 23, 2018
Title: 5T090 ACR Abstract: SLE–Clinical III: Translational Aspects (2832–2837)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Mycophenolate mofetil (MMF) is a commonly used medication to treat major organ involvement in SLE, specifically in patients with lupus nephritis. The safety and effectiveness of MMF therapy in SLE is well-known and determined by clinical response, but the systemic impact of MMF treatment on immune cellular subsets, cell activation and soluble mediator pathways in SLE remain ill-defined.
Methods: PBMCs and plasma samples from SLE patients not taking MMF (n=10) and SLE patients taking MMF (n=5) were studied. Subjects were matched by gender, ethnicity, age ± 5 years, medication use, and disease activity by SELENA-SLEDAI, and fulfilled the American College of Rheumatology (ACR) criteria for SLE classification. Using single cell proteomics by mass cytometry, PBMCs were clustered using 33 markers and cell heterogeneity was visualized using viSNE in Cytobank. Plasma cytokine levels were assessed by 51-plex xMAP assays and ELISAs. Flow cytometry was utilized to assess STAT3 phosphorylation and apoptosis of healthy control PBMCs (n=6) following treatment with IL-6 and MMF in vitro.
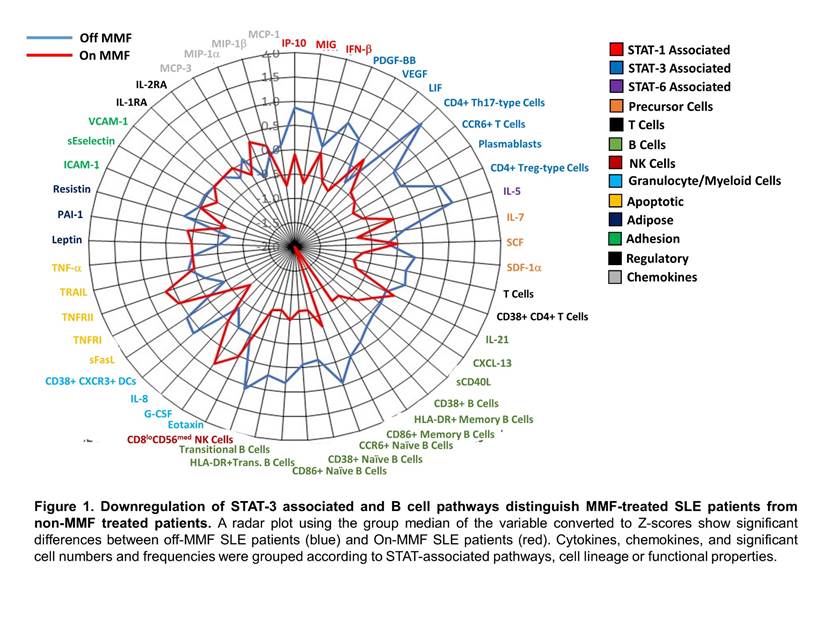
Results: Patients taking MMF had significant reductions in total numbers of transitional B cells (p=0.0077), plasmablasts (p=0.0480) and T cells (p=0.0486), specifically CD4+ Th17-type cells (p=0.0260) and CD4+ Treg-type cells (p=0.0469), compared to SLE patients not taking this medication. In addition, activation both of dendritic cells (p=0.0080) and B cells (p=0.04), specifically naïve (p=0.0127) and memory B cells (p=0.04), were reduced in patients taking MMF compared to non-MMF patients. MMF patients also had reduced levels of activated CD4+ T cells (p=0.0483). Plasma soluble mediators were decreased in MMF treated SLE patients including chemokines (MIG/CXCL9 and SDF-1a/CXCL12) and growth factors (VEGF-A and PDGF-BB) compared to non-MMF patients (P<0.05). Cytokines, chemokines and significant cell populations grouped by STAT-pathways, cell lineage and functional properties revealed significant modifications associated with the STAT3 and B cell pathways (Figure 1). Healthy PBMCs treated with IL-6 and MMF identified a significant downregulation of pSTAT3 following MMF addition (p=0.0313), but no alterations in pSTAT5 or caspase3/7 levels were observed following a 3 hour incubation.
Conclusion: Our results indicate that MMF suppressed STAT3 phosphorylation in response to IL-6 with associated decreases in antigen presentation, lymphocyte activation, and pro-inflammatory soluble mediators of SLE patients. Together these data suggest immunologic changes in the STAT3 pathway are critical for driving MMF disease remission in SLE patients.
To cite this abstract in AMA style:
Slight-Webb S, Guthridge JM, Chakravarty E, Chen H, Lu R, Bean KM, Maecker HT, Utz PJ, James JA. Diminished STAT-3 Phosphorylation and Associated Cell Pathways Characterize MMF-Treated Systemic Lupus Erythematosus Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/diminished-stat-3-phosphorylation-and-associated-cell-pathways-characterize-mmf-treated-systemic-lupus-erythematosus-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diminished-stat-3-phosphorylation-and-associated-cell-pathways-characterize-mmf-treated-systemic-lupus-erythematosus-patients/