Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) and spondyloarthritis (SpA) are immune-mediated inflammatory diseases (IMIDs) characterized by joint and spinal inflammation, leading to functional impairment. Their management requires close monitoring and effective communication between patients and healthcare providers. Digital health tools can help overcome current gaps in care by facilitating follow-up and interaction, especially within mixed care models that combine in-person and remote visits. Our aim was to evaluate the clinical implementation of a mixed care model using a digital tool called IMIDOC®, designed for both patients and healthcare professionals.
Methods: This prospective, multicenter study was conducted under the FIS PI22/00777 project. In its initial phase, IMIDOC was developed as a mobile app for patients and a web-based platform for clinicians. Patients can report health-related outcomes (PROs), clinical incidents, access educational content, and manage appointments and medication reminders. The clinician interface allows tracking of patient progress and timely intervention. In the clinical phase, 260 patients with RA or SpA receiving biological or targeted synthetic DMARDs (b/tsDMARDs) were scheduled for protocolized mixed follow-up for up to 12 months.
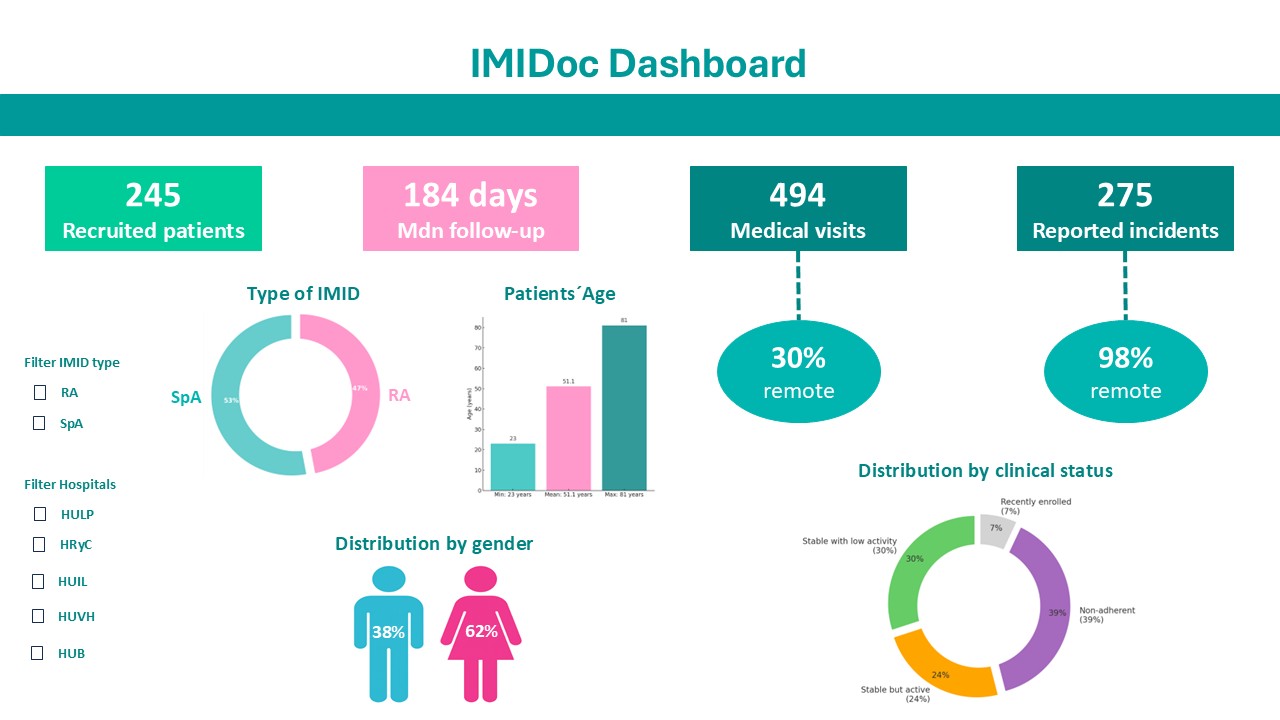
Results: Between April and October 2024, 245 patients were recruited (62% women; mean age 51.5 ±11.5 years; 47% RA, 53% SpA). The median follow-up time was 184 days (IQR 75–229). A total of 275 clinical incidents were reported (19% flares, 11% infections, 29% medication-related, 41% other), 98% of which were resolved remotely (77% via messaging, 23% via phone); only 2% required in-person visits. The median resolution time was 1 day (IQR 0–3). Among 494 medical visits, 29% were conducted remotely and 71% in person. A total of 15,011 PRO questionnaires were completed (6324 for RA, 8687 for SpA). In RA, the most frequent PROs were p-NAD (15%), p-NAT (14%), and p-VAS (14%); in SpA, p-NAD (11%), p-painful entheses (11%), and p-VAS (12%). Based on PRO-derived disease activity, 30% of patients were stable with low activity, 24% were stable but active, 39% were non-adherent, and 7% were recently enrolled. There were 609 consultations with educational materials, and at least 63% of patients accessed these resources, most commonly those related to joint self-assessment, the role of the rheumatologist, and early signs of disease. The app’s usability was rated 81 on the System Usability Scale (SUS).
Conclusion: The mixed care model supported by the IMIDOC tool represents a feasible and effective strategy for managing RA and SpA, combining digital and clinical components to enhance real-time monitoring, patient empowerment, and remote resolution of incidents
To cite this abstract in AMA style:
INIESTA-CHAMORRO J, Novella-Navarro M, Benavent D, BORREL-PAÑOS H, Michelena Vegas X, Bachiller J, García García V, ESPARTAL E, LOJO-OLIVEIRA L, Calvo-Aranda E, Rodríguez gago J, Bonilla G, Monjo Henry I, Navarro-Compan V, Arroyo J, de Miguel E, Díaz-Almirón M, ALEGRE c, GÓMEZ E, Plasencia-Rodríguez C. Digital Transformation in IMID Care: Results from the Implementation of IMIDOC [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/digital-transformation-in-imid-care-results-from-the-implementation-of-imidoc/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/digital-transformation-in-imid-care-results-from-the-implementation-of-imidoc/