Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Acceptance and Commitment Therapy (ACT), a form of guideline-recommended Cognitive Behavioral Therapy (CBT), has been empirically validated as a non-drug treatment for fibromyalgia (FM). However, clinical adoption of the therapy has been limited partly due to availability of qualified providers as a major barrier.
A smartphone-based, prescription digital application (FM-ACT) that has been recently cleared by the FDA helps address the access barrier by delivering self-guided ACT for treatment of FM symptoms. Its preliminary clinical benefits have been demonstrated in a pilot RCT.1 This report presents the results from PROSPER-FM (NCT05243511), a pivotal, prospective, multi-center, randomized controlled trial (RCT).
Methods: Individuals meeting 2016 FM diagnostic criteria were randomized to receive 12 weeks of FM-ACT or a digital symptom tracker control (ST) while remaining stable on any ongoing FM treatment(s). The FM-ACT intervention consists of 42 daily structured ACT lessons, mindfulness practices, and activities to encourage paced exercise and behavior change. The ST program offers daily symptom tracking and access to FM education materials. Participants were informed they would receive one of two potentially beneficial treatments that were being evaluated for clinical effectiveness.
The primary endpoint was Patient Global Impression of Change (PGIC). Secondary endpoints included the Revised Fibromyalgia Impact Questionnaire (FIQ-R), pain intensity, pain interference, and sleep interference. Endpoints were collected weekly through electronic patient-reported outcomes (ePROs).
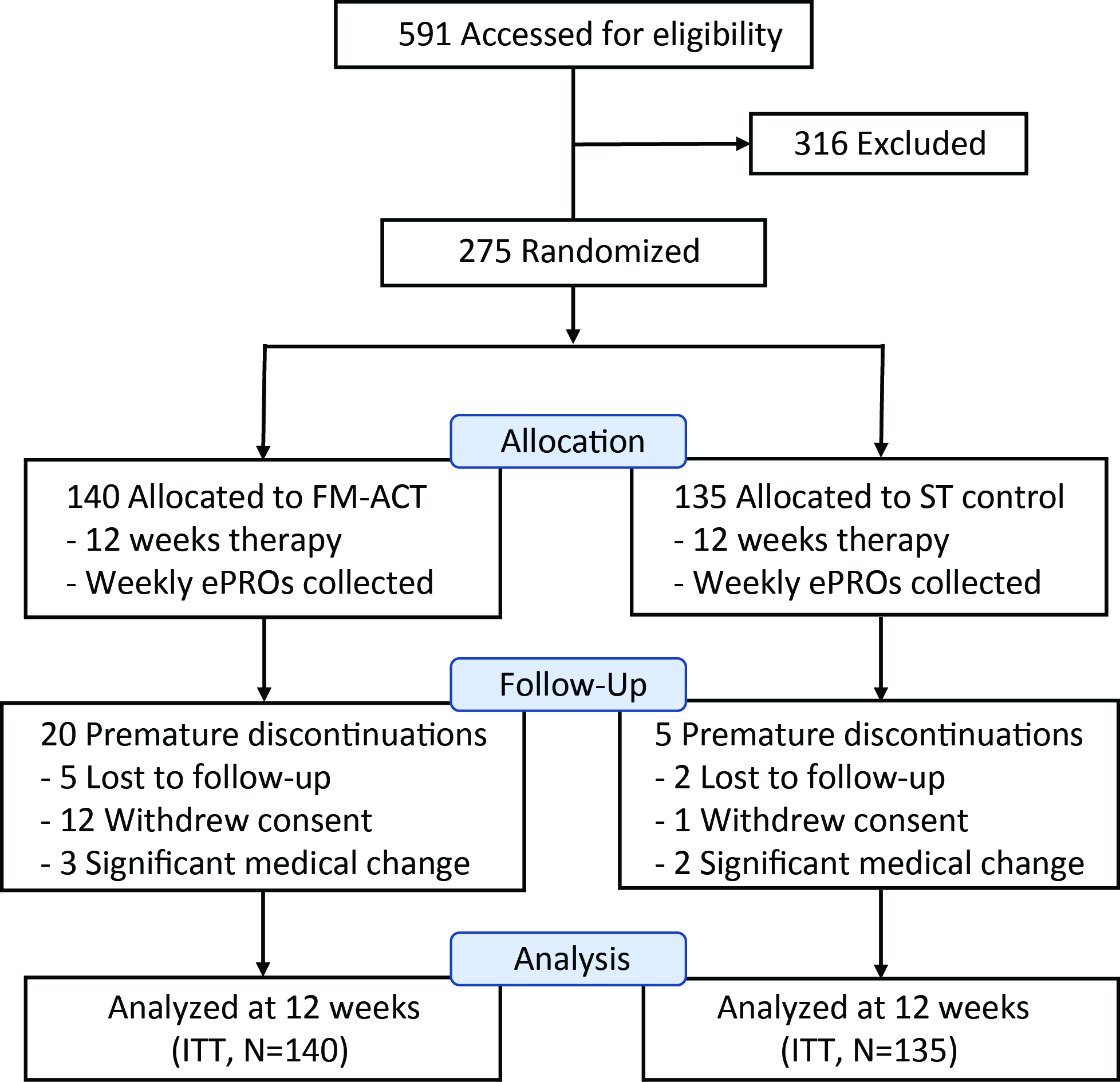
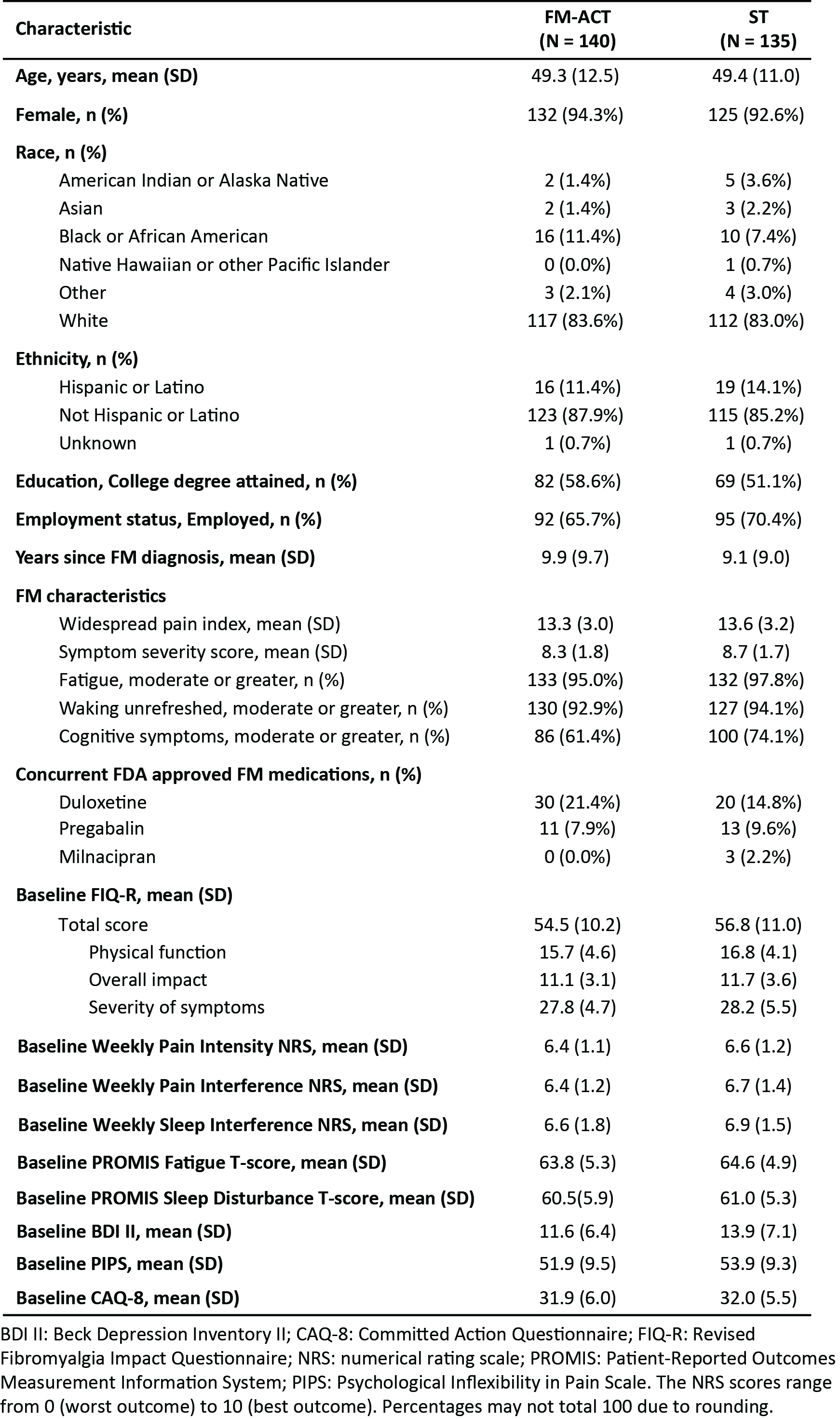
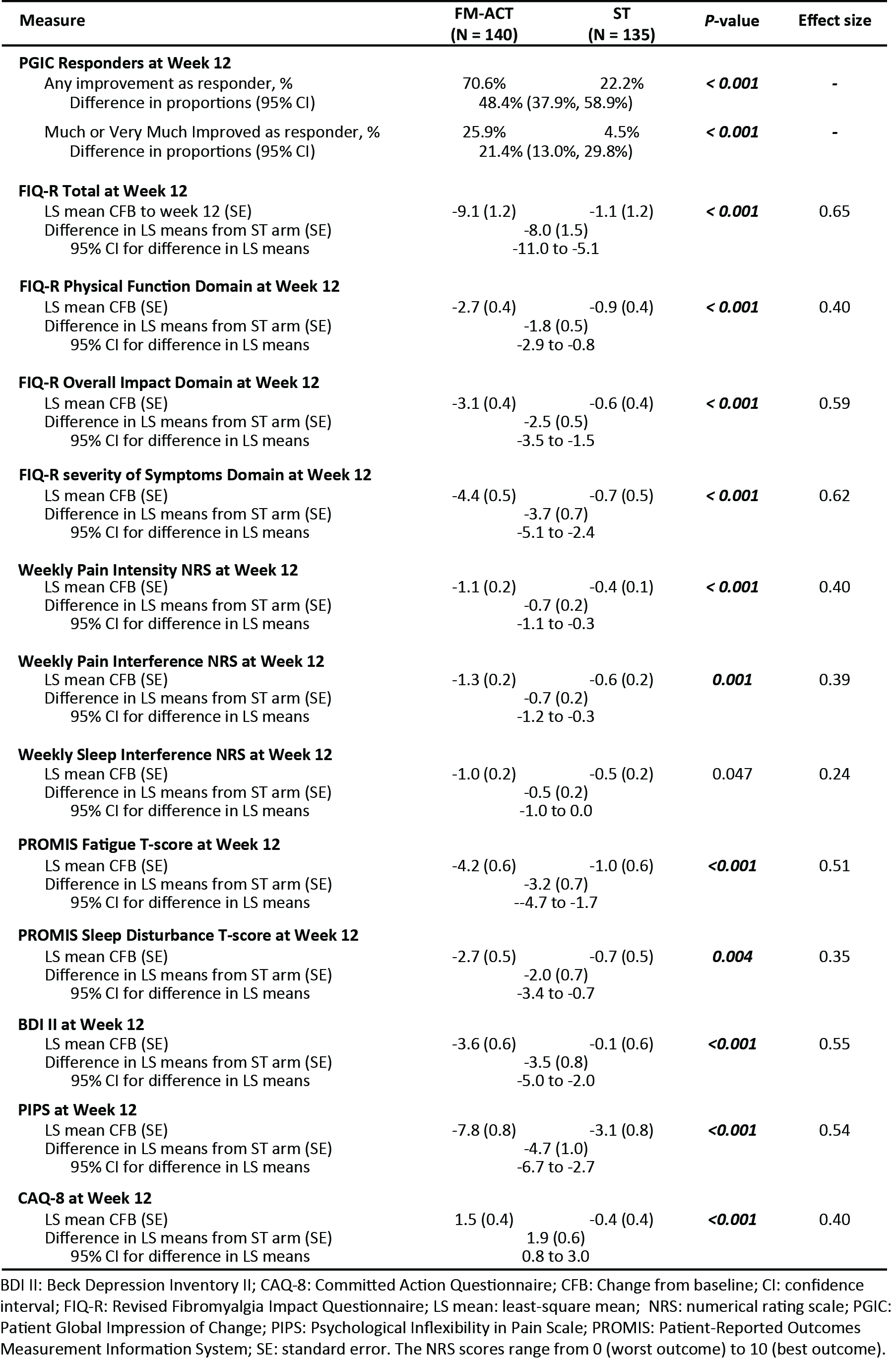
Results: A total of 275 participants were randomized (Fig 1, Table 1). Analysis results are summarized in Table 2. At week 12, 70.6% of the FM-ACT participants reported an improvement on PGIC, which was significantly greater than that reported by ST participants (22.2%) (p < 0.001). The FM-ACT arm exhibited a significantly greater post-treatment reduction on the FIQ-R total score as compared to the ST arm (p < 0.001, Effect Size = 0.65). FM-ACT was statistically superior to the control treatment on virtually all measures, including FIQ-R (total and domain-level scores), PGIC, pain intensity and interference, PROMIS Fatigue and Sleep Disturbance, Beck Depression Inventory II (BDI II), Psychological Inflexibility in Pain Scale (PIPS), and committed action (CAQ-8). No treatment related adverse events were observed.
Conclusion: Analysis of the PROSPER-FM pivotal trial supports the clinical benefits of FM-ACT in managing FM. This smartphone based digital therapeutic demonstrated statistical superiority over the control on patients’ wellbeing, FM symptoms, impact, and function, as well as common disorders associated with FM.
Validated digital ACT therapy provided by FM-ACT, combined with the low-risk safety profile of this device-based intervention, offers an important step forward in reaching and benefiting the broader FM population with a non-drug therapy.
References
- Catella et al. J Behav Med. Accepted for publication.
To cite this abstract in AMA style:
Gendreau M, Chadwick A, McCracken L, Williams D, Clauw D, Luciano J, Dai Y, Vega N, Ghalib Z, Guthrie K, Kraus A, Rosenbluth M, Zomnir J, Reddy D, arnold L. Digital Acceptance and Commitment Therapy Improves Fibromyalgia Outcomes: Results from a Pivotal, Multi-center, Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/digital-acceptance-and-commitment-therapy-improves-fibromyalgia-outcomes-results-from-a-pivotal-multi-center-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/digital-acceptance-and-commitment-therapy-improves-fibromyalgia-outcomes-results-from-a-pivotal-multi-center-randomized-controlled-trial/