Session Information
Date: Sunday, November 8, 2020
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Inhibition of TNFα has emerged as an effective therapeutic approach for many autoimmune/inflammatory diseases.While the efficacy and safety profile of the five FDA-approved TNFis (etanercept, adalimumab, certolizumab, golimumab, and infliximab) are comparable in rheumatoid arthritis (RA), there exist some intriguing differences among them. For example, one RA patient may respond to one TNFi but not the others; etanercept is generally ineffective for inflammatory bowel diseases or uveitis. Post-hoc data have also uncovered several unexpected sided effects of TNFis, such as +ANA, lupus-like diseases, demyelinating diseases, and pustular psoriasis, which probably can be partly attributed to the induction of type 1 interferons. Interestingly, certolizumab may be less likely to induce lupus-like diseases. The mechanisms mediating such discrepancies among TNFis are still poorly understood. The goal of this project is to examine whether TNFis have differential impacts on the expression of Th cytokines.
Methods: PBMC from healthy donors were stimulated with anti-CD3/anti-CD28 for 24 hours in the presence or absence of adalimumab, etanercept, or certolizumab for 24 hours. The expression of Th cytokines in PBMC or subsequently purified Th cells was analyzed with qPCR as well as RNA-seq. Flow cytometry was used to examine the binding of TNFis to membrane TNFα.
Results: Adalimumab and etanercept, but not certolizumab, inhibited the expression of IL-17A/F and IL-2 in Th cells within anti-CD3-stimulated PBMC (Figure 1). This discordant effect between adalimumab and certolizumab was not due to neutralization of soluble TNFα or their differential binding to membrane TNFα. Instead, the unique effect of adalimumab required cell-cell contacts between Th cells and non-Th cells and was not recapitulated by cross-linking of the Fc of adalimumab. RNA-seq analyses further revealed that adalimumab also suppressed the expression of other effector Th cytokines, including IFNγ, IL-21, and IL-9, but reciprocally induced strong type 1 interferon signals and the expression of co-inhibitory molecules CD96 and PVRIG in Th cells.
Conclusion: Adalimumab, but not certolizumab, inhibits the expression of Th cytokines independently of neutralizing soluble TNFα. This phenomenon is very likely mediated by type 1 interferon signals and CD96/PVRIG co-inhibition. Elucidating the mechanism mediating the unique effect of adalimumab very likely will uncover novel mechanisms of action of TNFis and improve their efficacy and safety.
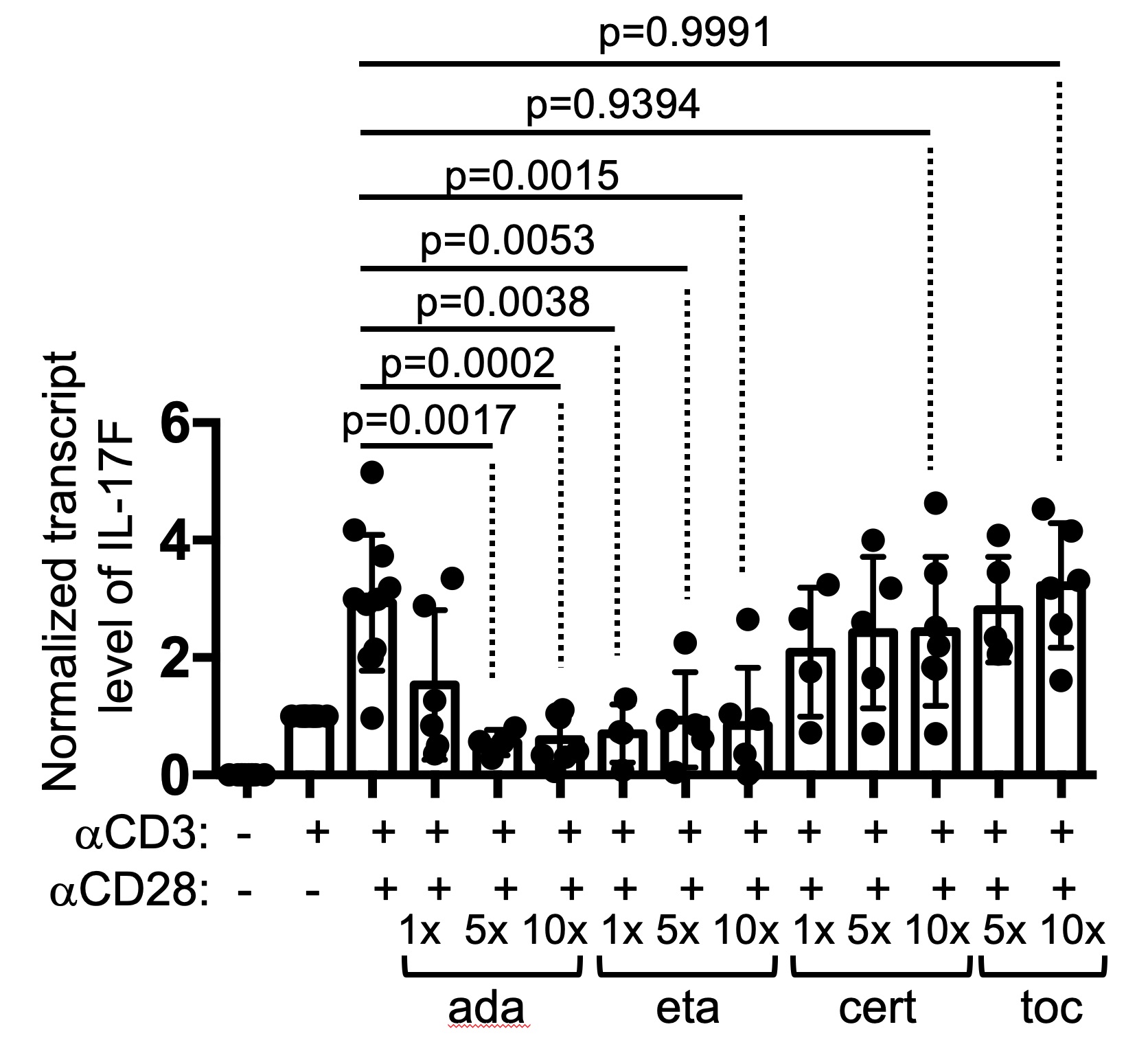 Figure 1. Drug-specific suppression of Th cytokine production by TNFis. PBMC of healthy donors were stimulated with anti-CD3 and/or anti-CD28 for 24 hours in the presence or absence of indicated TNFis at different concentrations (1X = 10 ug/ml for adalimumab, 25 ug/ml for etanercept, 10 ug/ml for certolizumab, and 20 ug/ml for tocilizumab). The transcript levels of IL-17F in the PBMC are shown. Statistical analysis was carried out with one-way ANOVA.
Figure 1. Drug-specific suppression of Th cytokine production by TNFis. PBMC of healthy donors were stimulated with anti-CD3 and/or anti-CD28 for 24 hours in the presence or absence of indicated TNFis at different concentrations (1X = 10 ug/ml for adalimumab, 25 ug/ml for etanercept, 10 ug/ml for certolizumab, and 20 ug/ml for tocilizumab). The transcript levels of IL-17F in the PBMC are shown. Statistical analysis was carried out with one-way ANOVA.
To cite this abstract in AMA style:
Ho C, Silva A, Ho I. Differential Impacts of TNFa Inhibitors on the Expression of Th Cytokines [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/differential-impacts-of-tnfa-inhibitors-on-the-expression-of-th-cytokines/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differential-impacts-of-tnfa-inhibitors-on-the-expression-of-th-cytokines/
