Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: There is no established consensus on defining the clinical course and the outcomes of systemic sclerosis-associated interstitial lung disease (SSc-ILD), both among experts and in the literature. Most proposed definitions of progression or severity of SSc-ILD are based on research data from idiopathic pulmonary fibrosis and are not fully validated for SSc-ILD. The aim of our study is to collect the available evidence about definitions of severity, progression and outcomes in SSc-ILD.
Methods: A systematic search of the literature was performed according to the PRISMA guidelines, to identify all papers including definitions of SSc-ILD severity, progression and outcome (PROSPERO registration CRD42022379254). Medline, Embase and Web of Science databases were searched up to December 31st, 2021.Randomized clinical trials, cross sectional or longitudinal studies were included if they focused on SSc or on cohorts in which data on SSc patients could be extracted, considered ILD as primary target (representing at least one of population, exposure, outcome) and included at least 10 adult patients. Exclusion criteria were papers not in English, non-human non-clinical studies, ILD onset as an outcome, literature reviews and no full-text availability.
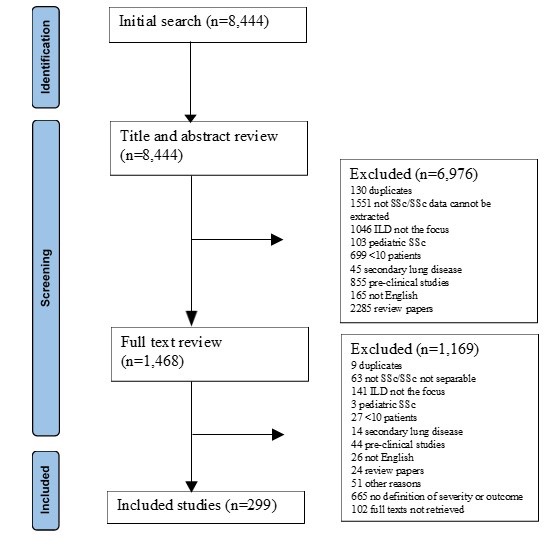
Results: Out of 8,444 papers identified by the primary literature search (Fig. 1), 299 original research manuscripts including 35’463 patients with SSc-ILD were finally selected. Mean age was 52 years (range from 32 to 66 years), 72.5 % were females. The mean disease duration ranged from 2 to 10 years.
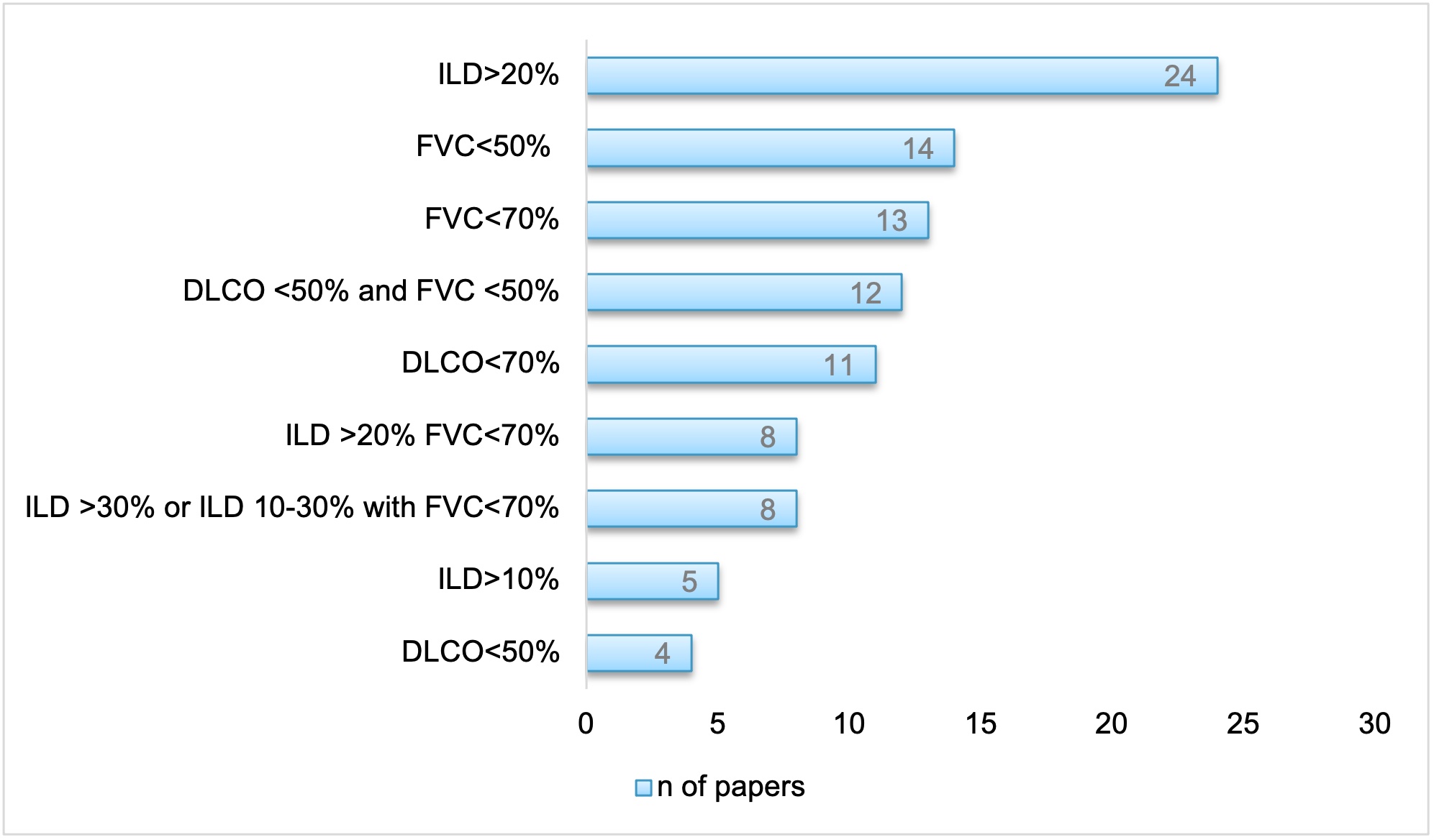
A definition of SSc-ILD severity was included in 138 (46%) papers (Fig. 2). Most papers (52%) used the extent of ILD on high resolution computed tomography (HRCT) to assess severity, mostly through visual quantification, either alone or in combination with pulmonary function tests (PFTs) data. The second most frequently used tool for severity was PFTs, based on forced vital capacity (FVC) in 30 (22%), on combination of FVC and diffusion of the lung for carbon oxide (DLCO) in 15 (11%) and on DLCO in 11 (8%) papers.
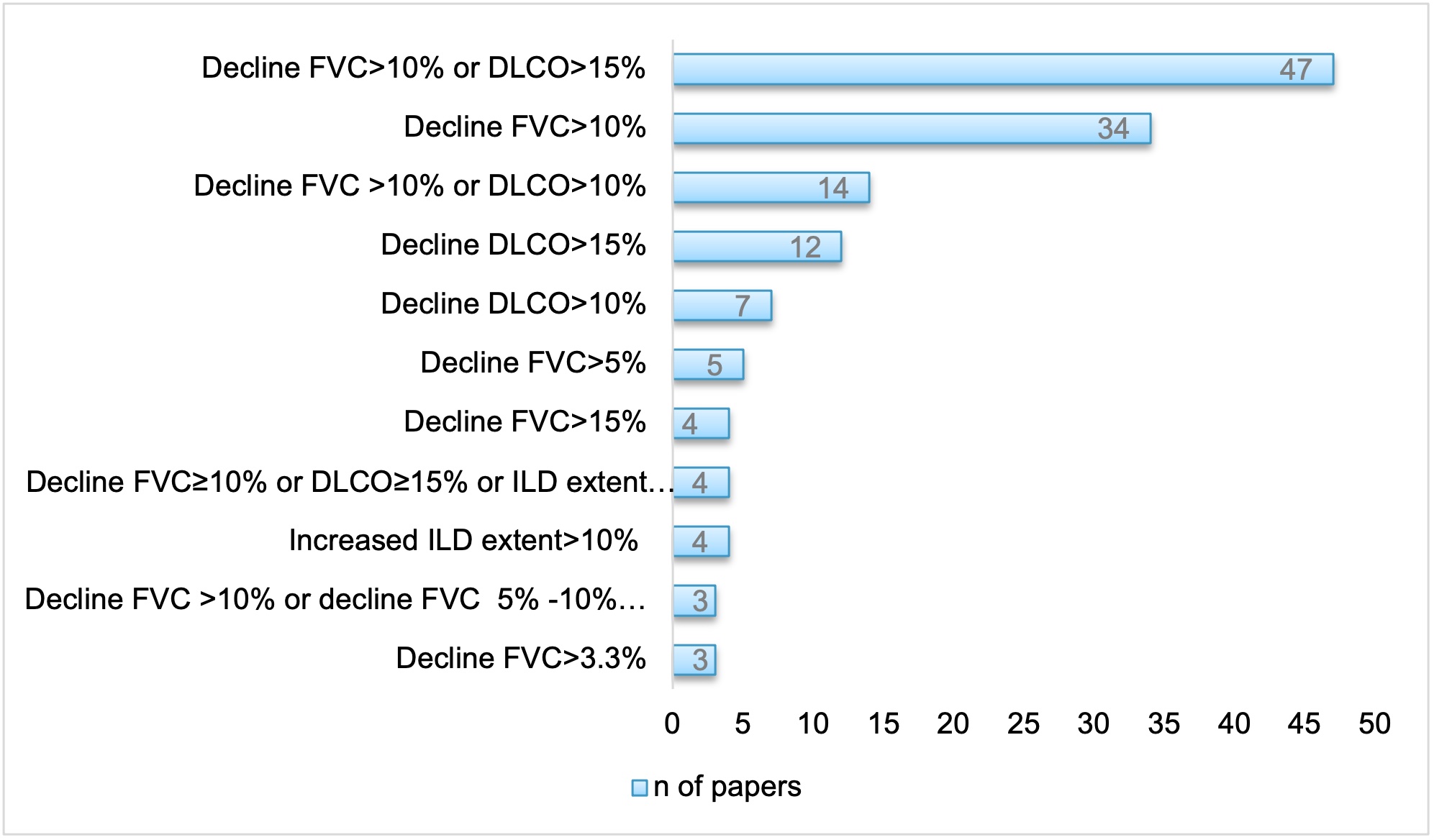
Sixty-four of 181 studies (35%) provided a definition of SSc-ILD progression referred to changes in FVC and DLCO, 51 (28%) in FVC, 23 (13%) in DLCO, 19 (10%) in ILD extent on HRCT, 7 (4%) to combination of PFTs and HRCT changes, 5 (3%) to combination of PFTs, clinical signs and HRCT data, 12 (7%) to other aspects (Fig.3). The timing to evaluate for SSc-ILD progression was also heterogeneous, including the re-assessment at 6 (5.5% papers), 12 (32.6 %), 24 (16.6%), 36 (10.5%), 60 (3.3%), or more than 60 months (7.2%).
The long-term outcomes recorded included mortality (131 papers, both ILD and non ILD-related), hospitalization (4 papers), end-stage ILD (5 papers), lung transplantation (2 papers) and infections (1 paper). Non-primarily ILD-related outcomes, such as malignancy, were also identified (4 papers).
Conclusion: our study showed large heterogeneity in definitions of SSc-ILD progression, severity, and outcome. This emphasizes the need to develop a standardized, consensus definition of severe SSc-ILD, to link a disease specific definition of progression as a surrogate outcome for clinical trials and clinical practice.
To cite this abstract in AMA style:
Petelytska l, Bonomi F, Cannistrà C, Fiorentini e, Peretti S, Torracchi S, Bernardini P, Coccia C, De Luca R, Economou A, Levani J, Matucci Cerinic m, Distler O, Bruni C. Different Definitions of Disease Severity, Progression and Outcomes in Systemic Sclerosis Associated Interstitial Lung Disease: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/different-definitions-of-disease-severity-progression-and-outcomes-in-systemic-sclerosis-associated-interstitial-lung-disease-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/different-definitions-of-disease-severity-progression-and-outcomes-in-systemic-sclerosis-associated-interstitial-lung-disease-a-systematic-literature-review/