Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
The clinical diagnosis of rheumatoid arthritis (RA) remains a challenge, making serological markers most interesting. The 2010 ACR/EULAR classification criteria for RA take into account two serological markers: anti-cyclic citrullinated peptide (ACPA) and rheumatoid factor (RF).
The objective of this study is to evaluate the diagnostic performance of 6 RF and 7 ACPA assays in a secondary care hospital
Methods:
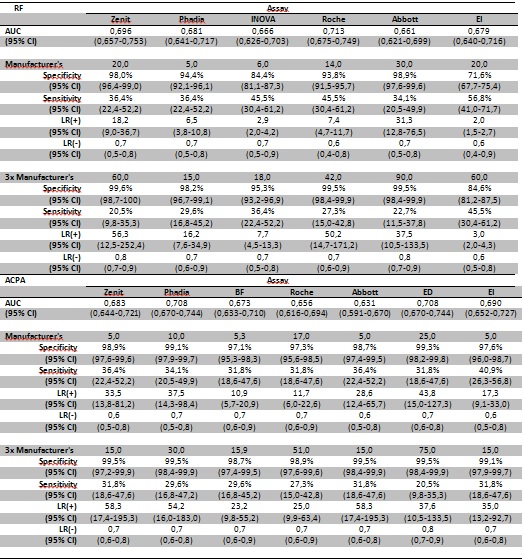
From January 2014 to June 2015, all unique patients who for the first time underwent laboratory testing for a rheumatologic disease at the OLV hospital Aalst were included. Review of the medical records was done and the diagnosis was registered and reviewed by the consulting rheumatologist. Patients were categorized into three groups: RA, rheumatologic disease control group (RDCG) and disease control group (DCG). For all RA patients the fulfillment of the ACR classification criteria (1987 and 2010) was checked. Six commercial RF assays (Zenit, Phadia, Inova, Roche, Abbott, Euroimmun (EI)) and 7 commercial ACPA assays (Zenit, Phadia, BIOFlash Inova (BF), Roche, Abbott, EuroDiagnostica (ED), EI) were tested and diagnostic performance (sensitivity, specificity, receiver operating characteristics (ROC) curve analysis, likelihood ratio (LR)) evaluated.
Results:
We included 594 patients: 44 (7,4%) RA, 247 (41,6%) RDGC and 225 (37,9%) DCG. For 78 (13,1%) the diagnosis remained undifferentiated. Most of the included RA patients fulfilled the ACR classification criteria (94,3% 1987; 78.56% 2010).
Sensitivities for RF range from 35,7-44,3% and for ACPA from 35,7-41,4% (Table 1), lower than normally described. Demographic patient features, like older patients, could be partly responsible for this low RF/ACPA positivity. The low false positive reactivities in the control patient cohorts are also quite remarkable for RF, as RF is historically known as a more aspecific serological RA marker. Since the control group consists of a primary diagnostic, consecutive rheumatological population, they represent the real life setting in this secondary care hospital, reflecting the true diagnostic usefulness of the RF/ACPA analyses. A negative serological test result will not exclude RA. For RF the importance of a weak positive result (1-3 times cut-off value) depends on the assay used. For ACPA a weak positive result is of diagnostic importance. Strong positive RF or ACPA results can aid in the diagnosis of RA as they have a high LR for RA (>10 for all assays), except for two RF assays (from Inova and Euroimmun).
Conclusion:
The aid of the serological reactivities in the diagnosis of RA is dependent on the choice of the RF (especially) or ACPA assay used due to the lack of harmonization. Demographic features of the patient population and the possible low serological positive RA pretest probability will also influence the usefulness of these serological tests.
To cite this abstract in AMA style:
Van Hoovels L, Jacobs J, Vander Cruyssen B, Van den Bremt S, Verschueren P, Bossuyt X. Diagnostic Performance of 7 Different Anti-Cyclic Citrullinated Peptide Antibody and 6 Rheumatoid Factor Assays in a Primary Diagnostic, Consecutive Rheumatological Population in a Secondary Care Hospital [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/diagnostic-performance-of-7-different-anti-cyclic-citrullinated-peptide-antibody-and-6-rheumatoid-factor-assays-in-a-primary-diagnostic-consecutive-rheumatological-population-in-a-secondary-care-hosp/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diagnostic-performance-of-7-different-anti-cyclic-citrullinated-peptide-antibody-and-6-rheumatoid-factor-assays-in-a-primary-diagnostic-consecutive-rheumatological-population-in-a-secondary-care-hosp/