Session Information
Date: Tuesday, November 14, 2023
Title: (2177–2194) Sjögren’s Syndrome – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Sjögren disease (Sjo) is an autoimmune disease characterized by the infiltration of exocrine glands by immune cells, especially salivary glands (SG). SG epithelial cells (SGEC) are involved in Sjo pathogenesis, mostly through their crosstalk with immune cells. The primary 2D culture of SGEC derived from Sjo patients has been very useful to better understand Sjo pathogenesis but does not recapitulate the complexity of the SG and lacks epithelial readout, highlighting the necessity to develop new relevant models. Our objective was to develop and characterize differentiated organoids of SGEC derived from minor salivary gland (MSG) of Sjo patients and controls.
Methods: We included Sjo patients fulfilling the ACR/EULAR 2016 criteria and controls with sicca syndrome. MSG were firstly dissociated enzymatically, then encapsulated in Matrigel© and cultured in Growth Expansion Medium (GEM) containing growth factors, until organoids formation. Organoids were passaged every 7-11 days for self-renewal and cultured in a Differentiation Medium (DM) inhibiting the NOTCH pathway, to develop mature organoids. Proliferation was assessed by cell counting and SGEC markers were investigated by RT-qPCR and immunofluorescence.
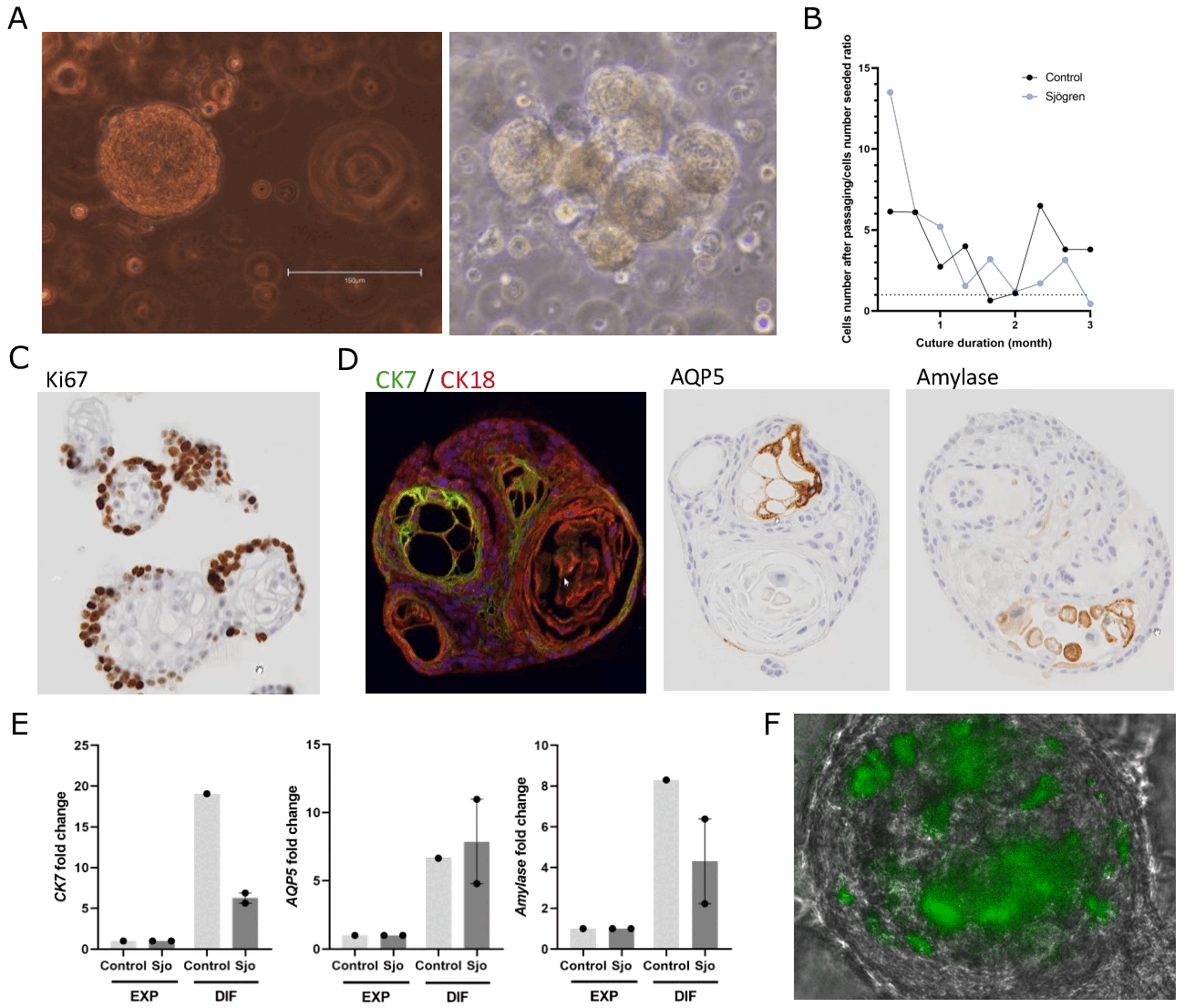
Results: Ten patients were included: 8 controls and 2 Sjo patients. Organoids with an average size of 100-200 µm were forming and differentiating in both patients and controls (Figure 1A). The mean culture duration was 2.7 ± 1.7 months and was continued for up to 6 months. The self-renewal capacity of organoids in expansion condition was comparable between the two groups, as evidenced by comparable growth curves for the 2 groups (Figure 1B) and the high expression of the Ki67 marker in the periphery of organoids, arguing an active cell proliferation (Figure 1C). We then differentiated the organoids. Mature organoids highly expressed the ductal markers CK7/CK18 and, to a lesser degree, the acinar markers AQP5 and α-amylase, in both Sjo patients and controls (Figure 1D). We confirmed these results in RNA expression, showing an enhanced expression of acinar and ductal markers with DM compared to GEM (Figure 1E). Last, we have performed one preliminary experiment in which we have been able to develop immune-organoids by adding PBMC in the organoids (Figure 1F). Work is still in progress to assess the impact of immune cell infiltrate on organoid characteristics.
Conclusion: We established a culture system of SG organoids from MSG in Sjo patients and controls with long term expansion and maturation markers expression. Additional investigations are ongoing to assess the functionality of the organoids and to develop immune-organoids for assessing the impact of adding immune cells in the system. Bibliography 1. Pringle S, et al. Arthritis Rheumatol. 71(1):133‑42.
(A) SG organoids in expansion and differentiation captured by photonic microscopy. (B) SGEC expansion by the ratio between cell count at seeding and at passaging. (C) Ki67 expression in organoids cultured in GEM. (D) CK7/CK18 (ductal), AQP5 and α-amylase (acinar) expression in organoids cultured in DM. (E) CK7, AQP5, and α-amylase RNA expression in SG organoids cultures in GEM and DM (n=3). (F) Infiltration of SG organoid by CFSE-labelled PBMC.
To cite this abstract in AMA style:
Meudec L, Goudarzi N, Pascaud J, Jaulin F, Mariette X, Nocturne G. Development of Salivary Gland Organoids to Study Sjögren Syndrome [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/development-of-salivary-gland-organoids-to-study-sjogren-syndrome/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-salivary-gland-organoids-to-study-sjogren-syndrome/