Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Pediatric Rheumatology – Clinical I: Connective Tissue Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: There is a paucity of prospective clinical trials evaluating treatments for juvenile dermatomyositis (JDM). Consensus treatment plans (CTPs) are designed to facilitate comparative effectiveness studies between treatments. The biologic committee of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JDM workgroup developed biologic disease modifying anti-rheumatic drug (DMARD) CTPs for patients with refractory moderate JDM, the ultimate goal of which is to determine effective and safe biologic treatments.
Methods: The JDM biologic committee convened in 2011. The initial CTP development process was described in 2017 by Spencer et al. (https://doi.org/10.1186/s12969-017-0174-0). This work was the foundation for the 2019 CARRA JDM biologic meeting, where key elements lacking consensus were discussed. A core group of 20 stakeholders including pediatric rheumatologists, trainees, and parents developed a survey to assess current treatment escalation practices for moderately severe JDM. The cases and questions included in the survey were developed using a consensus framework, requiring at least 80% agreement. The resulting survey was distributed online in 2020 and completed by 121 self-selected CARRA members interested in JDM. The results of the survey informed the final CTP, which was distributed to 100 randomly selected CARRA members for evaluation from November 2021-March 2022.
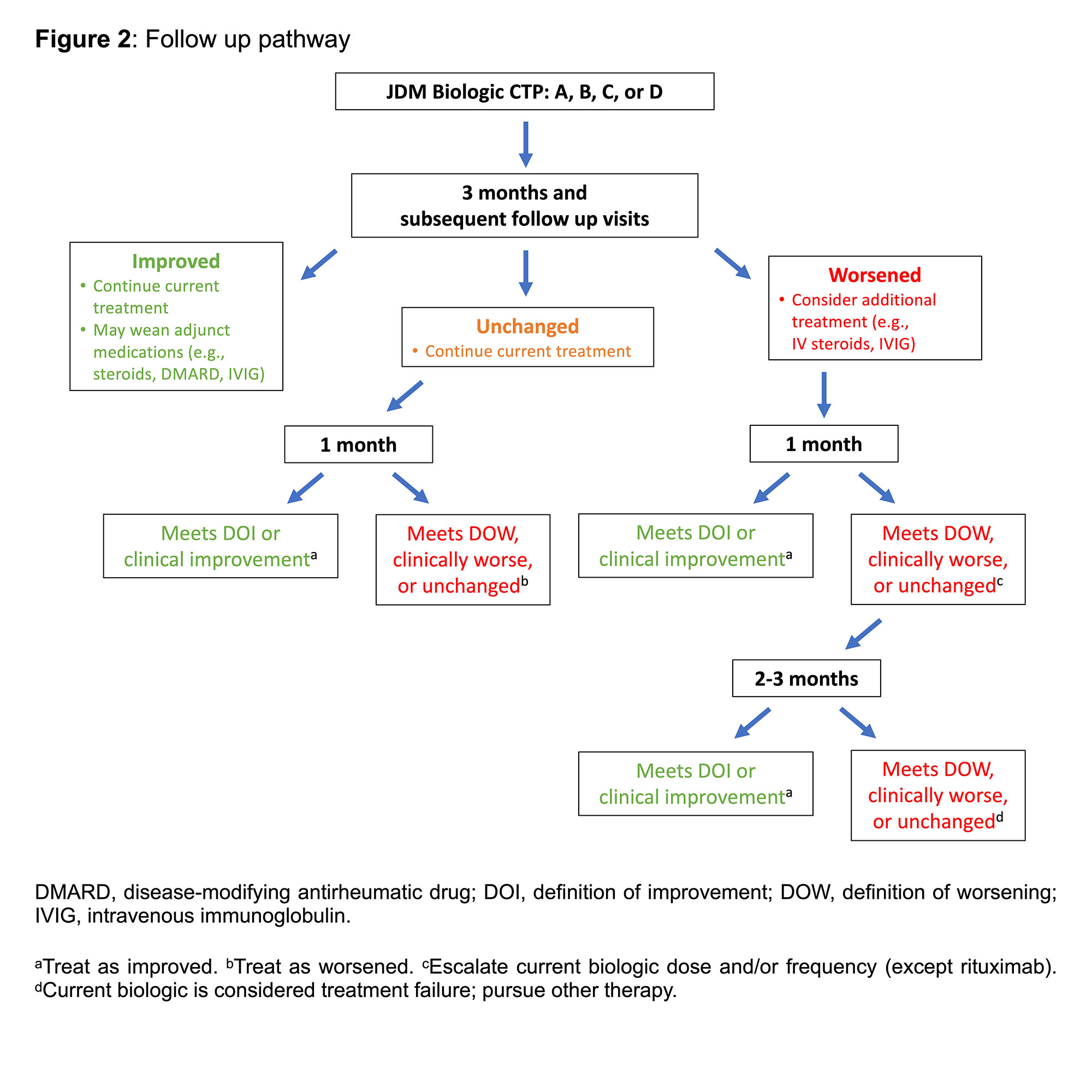
Results: Four biologic CTPs for refractory moderately severe JDM were proposed: TNF-alpha inhibitor (adalimumab or infliximab), abatacept, rituximab, and tocilizumab (Figure 1). Each CTP has different options for dosing and/or route. Eligibility criteria as well as baseline and follow up assessments were determined. Established definitions for improvement, worsening, and clinically inactive disease were included. A pathway for clinical assessment and follow up was outlined (Figure 2). There were 91 responses to the survey; of those, 10 opted out, 2 were not pediatric rheumatology providers practicing in North America, and 3 did not treat patients with JDM. Among the 72 respondents, consensus was achieved for the proposed CTPs (93% [67/72]) as well as for patient characteristics, assessments, definitions of clinical status, and follow up. By weighted average, respondents indicated that they would most likely use rituximab followed by abatacept, TNF-alpha inhibitor, and tocilizumab.
Conclusion: CTPs for the use of biologic treatments in refractory moderate JDM were achieved using consensus methodology. The implementation of the CTPs will lay the groundwork for registry-based prospective comparative effectiveness studies.
Acknowledgements: The authors wish to acknowledge CARRA and the ongoing Arthritis Foundation financial support of CARRA; the Intramural Research Program of NIAMS/NIH.
To cite this abstract in AMA style:
Sherman M, Kim H, Tarvin S. Development of CARRA Biologic Consensus Treatment Plans for Management of Refractory Moderate Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-of-carra-biologic-consensus-treatment-plans-for-management-of-refractory-moderate-juvenile-dermatomyositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-carra-biologic-consensus-treatment-plans-for-management-of-refractory-moderate-juvenile-dermatomyositis/