Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: As part of a larger study developing classification criteria for axial disease in children with spondyloarthritis (SpA), the objective of this project phase was to identify the strength of association of clinical and imaging features with expert classification.
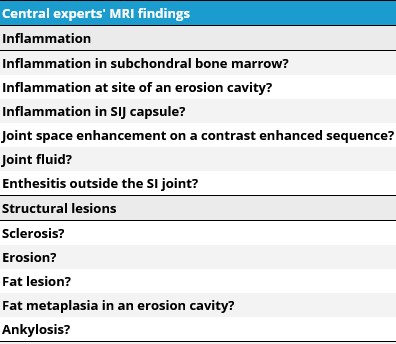
Methods: We identified key clinical and imaging features to classify children with axial disease through an iterative process that included international Delphi exercise for item generation, systematic literature review, and an item reduction exercise. Case report forms and pelvic imaging (MRI and, if available, radiograph) of children with SpA and suspected axial disease were collected from 14 international centers. All imaging was rated by ≥2 of 7 radiologists from a central imaging team. The central imaging assessment consisted of presence or absence of the lesions shown in Figure 1. Fourteen juvenile SpA experts completed two global assessments using: (1) clinical features and local radiograph report (if available) and (2) clinical factors plus central imaging team assessment (MRI ± radiograph). Each SpA expert rated their confidence that the case was “juvenile SpA with axial disease” on a scale with anchors -3 (very unlikely) to +3 (very likely). High confidence in expert assessment was defined as ≤-2 or ≥+2. Each case was reviewed by 3 experts and consensus was defined as ≥2/3. Strength of association was measured using odds ratios.
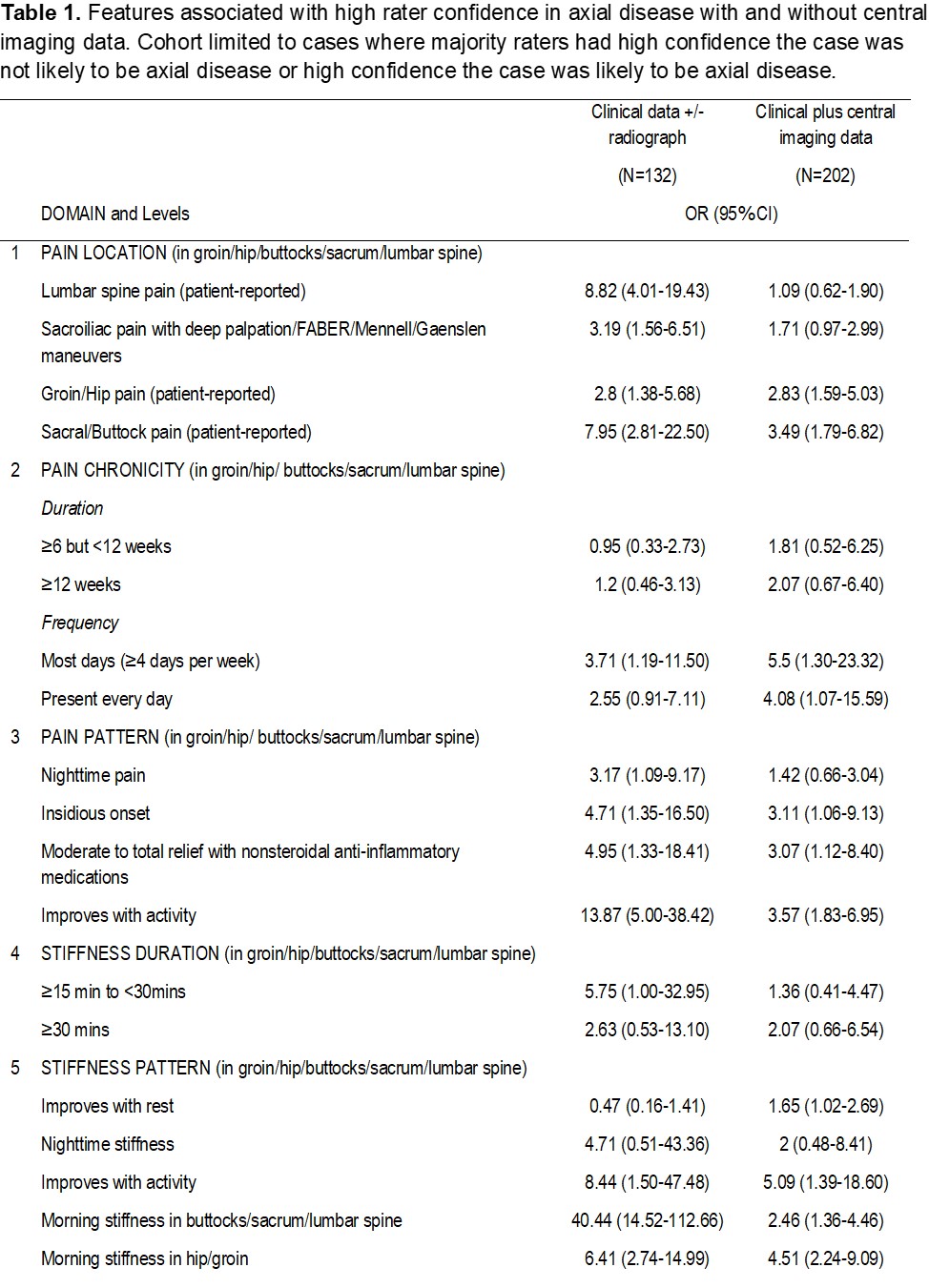
Results: Eight domains were chosen for inclusion in the preliminary classification criteria (Table 1). 304 cases were assessed by the experts, of which 208 (68.4%) had findings typical of axial juvenile SpA by the central imaging team. Expert consensus was achieved on 132 (43%) and 202 (66%) of cases with clinical factors (± radiograph) or clinical factors plus central imaging assessment. With central imaging data, the presence of HLA-B27, pain most days, and morning stiffness in the hip/groin were most highly associated with rater confidence in presence of axial disease (Table 1). After the addition of central imaging data, several factors lost their significant association with rater confidence in presence of axial disease, including patient-reported lumbar spinal pain, sacroiliac pain on examination, and nighttime pain. Adding central imaging data affected the consensus assessment of high confidence in the presence/absence of axial disease for 55% (168/304) of cases. The addition of central imaging data facilitated achievement of a majority consensus on an additional 113 cases but lost consensus on 37; of the 113 cases gaining consensus, 43 and 70 reached consensus with high confidence that the case was or was not likely axial disease, respectively. For 18 (5.9%) cases, it changed the directionality of high confidence; 5 cases from axial disease present to axial disease absent and 13 cases from not axial disease to axial disease present.
Conclusion: Imaging significantly impacts the assessment of the presence/absence of axial disease in juvenile SpA and the association of several clinical domains with axial disease. In addition to imaging, HLA-B27 presence, pain experienced most days, and stiffness in the hip and groin are significantly associated with high rater confidence in axial disease.
To cite this abstract in AMA style:
Weiss P, Brandon T, Aggarwal A, Burgos-Vargas R, Colbert R, Horneff G, Joos R, Laxer R, Minden K, Ravelli A, Ruperto N, Smith J, Stoll M, Tse S, Van den Bosch F, Maksymowych W, Lambert R, Biko D, Chauvin N, Francavilla M, Jaremko J, Herregods N, Kasapcopur O, YILDIZ M, Hendry A. Development of Candidate Criteria for Axial Disease in Juvenile Spondyloarthritis: An International Collaboration [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/development-of-candidate-criteria-for-axial-disease-in-juvenile-spondyloarthritis-an-international-collaboration-2/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-candidate-criteria-for-axial-disease-in-juvenile-spondyloarthritis-an-international-collaboration-2/