Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Gout is a multifactorial inflammatory disease in which patients experience a wide range of signs and symptoms, including flares, inflammatory arthritis, tophi and disability. Most assessments of gout and response to urate-lowering therapy (ULT) have focused primarily on the ability to lower serum urate and decrease the frequency of flares. Recognition that assessment of ULT and other treatments for gout could be facilitated by endpoints that more closely reflect the multidimensional impact of the disease has prompted an interest in developing composite measures, although there is no consensus on the most appropriate composite measure to employ. The goal of this research was to develop an evidence-based gout multivariable improvement measure (GMIM) that captures the spectrum of gout manifestations and is sensitive to change.
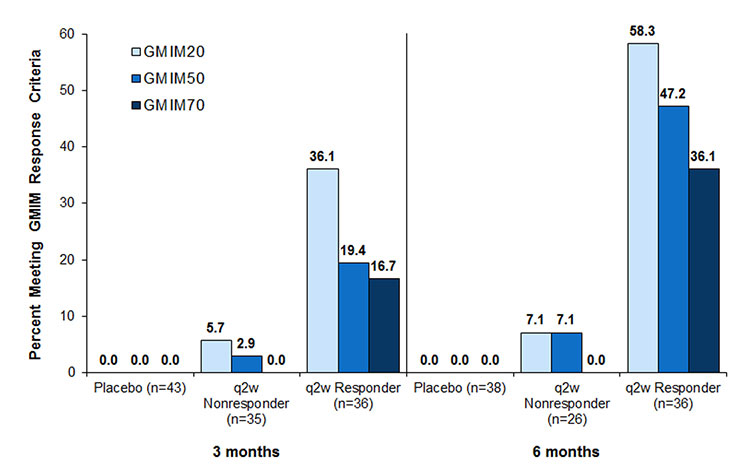
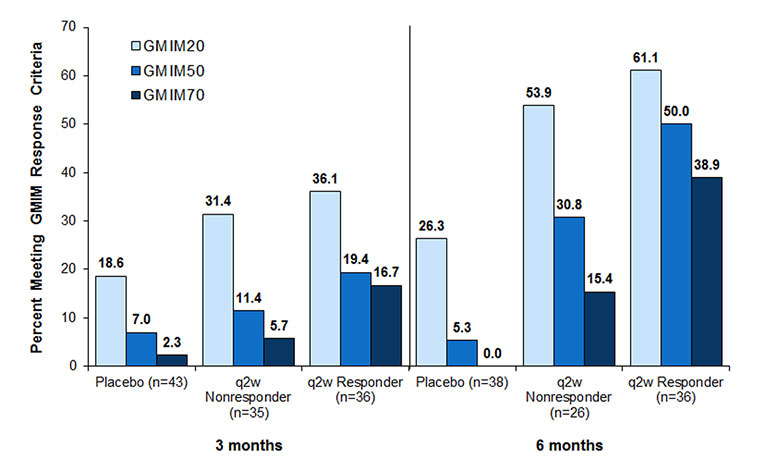
Methods: Databases from patients with chronic refractory gout who participated in two randomized 6-month clinical trials (RCTs) of pegloticase were reviewed. Sub-sets who had persistent urate lowering (responders) to biweekly pegloticase (n=36) and those who had only transient urate lowering (non-responders, n=49) were identified and compared to those who received placebo (n=43). Initially individual patients were assessed for achievement of previously reported criteria for remission: serum urate < 6 mg/dL, absence of tophi and flares, patient global assessment, and pain (each < 2 on a 10-point visual analog scale). A repeated measures mixed effects model controlling for repeated observations with backward elimination using data from these patients was employed to determine the model components that best correlated with time to maximum benefit. This analysis resulted in the addition of swollen and tender joints to the outcome measure. In order to assess the degree of improvement, each subject was scored based upon a serum urate < 6 mg/dL and 20, 50 or 70% improvement in ≥4 of the 6 clinical evaluations and termed GMIM20, 50, 70.
Results: GMIM was able to capture gradation of change in the treated populations and also distinguish responses in those with persistent versus those with transient urate lowering and subjects treated with placebo (Figure 1). At 3 and 6 months, achievement of GMIM20, 50 and 70 in persistent responders occurred significantly more often vs placebo and versus non-responders without persistent urate lowering. By eliminating the requirement for urate < 6mg/dL, the improvement in clinical features could be assessed (Figure 2). Improvement greater than that noted in subjects receiving placebo could be discerned in both responders and non-responder to pegloticase. Sensitivity analysis indicated that flares and pain contributed minimally to the model.
Conclusion: GMIM effectively captures changes in disease severity in response to treatment in patients with advanced gout treated with pegloticase. GMIM20,50,70 may serve as an evidence-based tool for assessment of the quality of response to therapies in subjects with gout in medical practice or in clinical trials.
To cite this abstract in AMA style:
Schlesinger N, Edwards N, Yeo A, Lipsky P. Development of a Multivariable Improvement Measure for Gout [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/development-of-a-multivariable-improvement-measure-for-gout/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-multivariable-improvement-measure-for-gout/