Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Black and Latinx individuals with autoimmune and inflammatory rheumatic diseases (AIRD) face disproportionately higher risks of severe COVID-19 outcomes. Despite this elevated risk, vaccine uptake remains low in these populations. Interventions that leverage narrative communication and homophily may promote behavior change. We developed a culturally and linguistically tailored, storytelling intervention designed to support a future randomized controlled trial (RCT) of COVID-19 vaccine uptake in Black and Latinx patients with AIRD.
Methods: Intervention development was guided by a conceptual model (Figure 1) created by the study team, which included two community investigators and a representative from a patient advocacy organization. Patients with AIRD from two academic rheumatology clinics in Alabama and Massachusetts and from a patient advocacy organization were recruited as storytellers. Storytellers, selected based on congruence with anticipated background of future RCT participants, signed a media release form and were compensated for their time. Semi-structured in-person or virtual interviews elicited storytellers’ experiences with COVID-19 vaccination, including barriers (e.g., access issues, safety concerns) and facilitators (e.g., trust in the healthcare system, vaccination health benefit) to vaccination. Filmed interviews were split into segments to identify key themes. Each segment was rated from 1 (lowest) to 5 (highest) for emotional authenticity, persuasiveness, content accuracy, and overall impression. The highest-rated segments discussing desired messages were compiled into a draft English-language video, which underwent structured review. A brief introduction featuring a rheumatologist was recorded to provide context for the audience. A professional videographer combined the introduction with the highest-rated storytelling segments, added titles, subtitles, and music to produce the final video. The Spanish-language footage was reviewed and edited to select storytelling segments with the same messages as those in the English-language video (Figure 2).
Results: Black (Nf4) and Latinx (Nf4) individuals with AIRD participated in structured ~30 minutes interviews in English or Spanish. Eight study team members reviewed and rated 165 minutes of footage in English and six Spanish-fluent study team members reviewed and rated 131 minutes of Spanish-language footage. The ~10 minutes draft English-language video was edited to ~4 minutes after the second round of review. Table 1 shows key broad themes, subthemes, and second round of review rating scores for each clip selected for the final videos. Both English and Spanish-language videos were edited to ~4 minutes (Figure 2).
Conclusion: Guided by the storytelling narrative communication theory and a structured iterative editing process, we produced two culturally tailored storytelling videos in English and Spanish that can be tested, together with patient navigation, as an approach to improve COVID-19 vaccination rates in Black and Latinx individuals with AIRD. Our robust approach to intervention development is scalable and adaptable to other preventative care interventions.
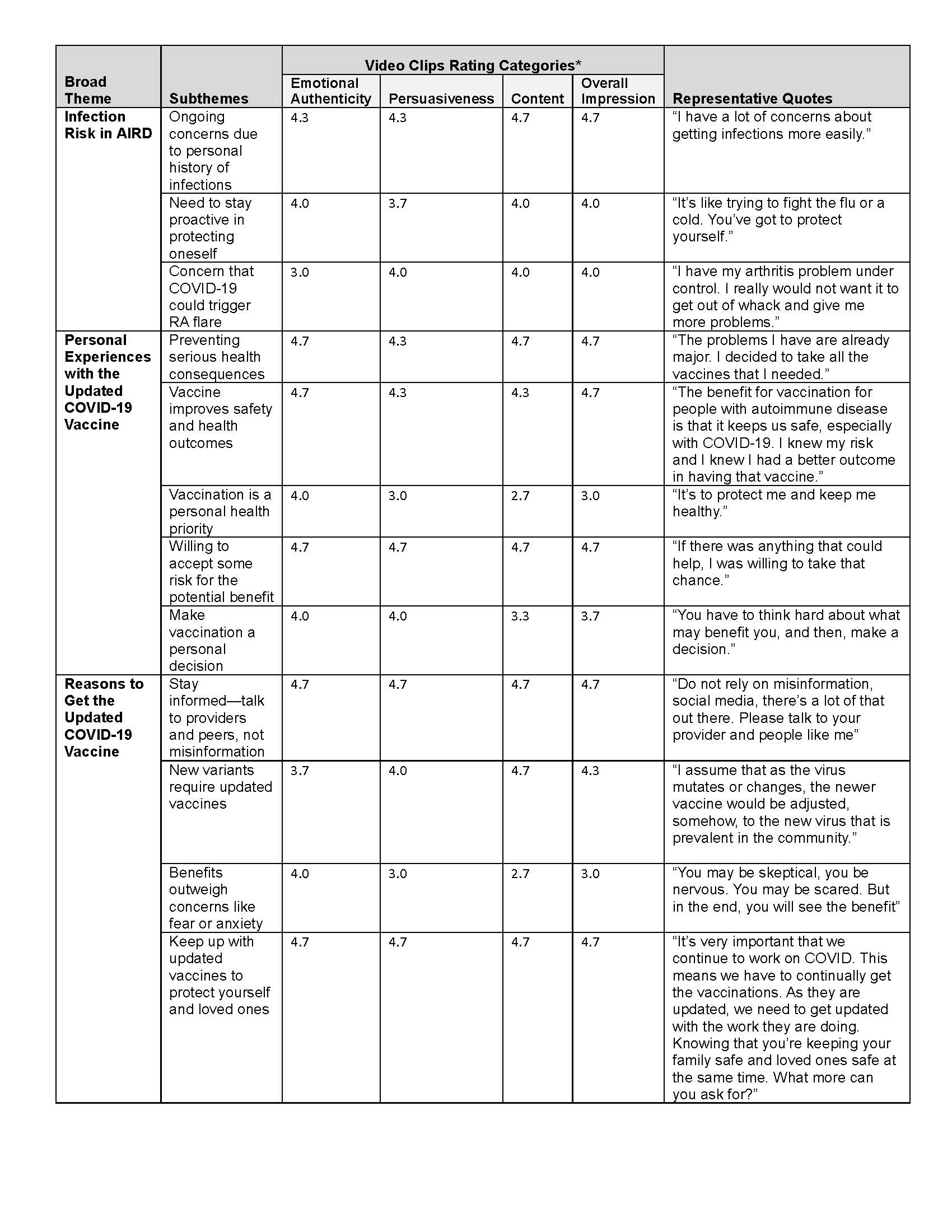 Table 1. Key overarching themes, subthemes, and rating scores for each clip represented in final storytelling video.
Table 1. Key overarching themes, subthemes, and rating scores for each clip represented in final storytelling video.
.jpg) Figure 1. Conceptual Model for the Multi-modal Intervention Design.
Figure 1. Conceptual Model for the Multi-modal Intervention Design.
.jpg) Figure 2: Storytellers and final topics chosen to promote the updated COVID vaccination in Black and Latinx patients with AIRD in English and Spanish.
Figure 2: Storytellers and final topics chosen to promote the updated COVID vaccination in Black and Latinx patients with AIRD in English and Spanish.
To cite this abstract in AMA style:
Danila M, Jackson L, Ford S, Wilkenson M, Alexander T, Allison J, Feldman C, Jenoure F, Kay J, Lemon S, Saag K, Salomon K, Venkatachalam S. Development of a Culturally-Tailored Storytelling Intervention to Improve COVID-19 Vaccine Uptake in Black and Latinx Patients with Autoimmune and Inflammatory Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-of-a-culturally-tailored-storytelling-intervention-to-improve-covid-19-vaccine-uptake-in-black-and-latinx-patients-with-autoimmune-and-inflammatory-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-culturally-tailored-storytelling-intervention-to-improve-covid-19-vaccine-uptake-in-black-and-latinx-patients-with-autoimmune-and-inflammatory-rheumatic-diseases/
