Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: ANCA-associated vasculitis (AAV) care has evolved over the last two decades with several approaches to remission maintenance. However, these approaches have trade-offs regarding the risk of flare, infection, end-stage renal disease (ESRD), and other outcomes which impact morbidity, mortality, and costs. We developed a simulation model to project clinical outcomes in individuals with specific disease features, which can be used to inform clinical decision-making and identify uncertainties that strongly affect outcomes.
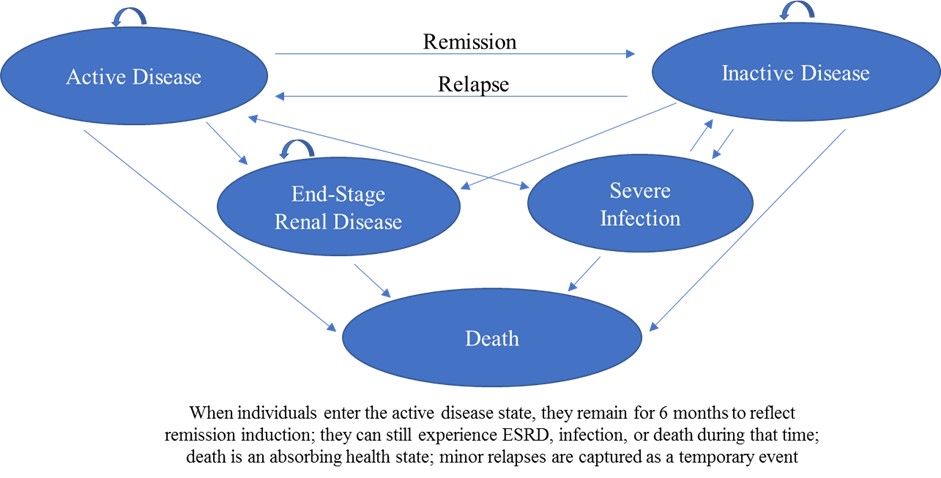
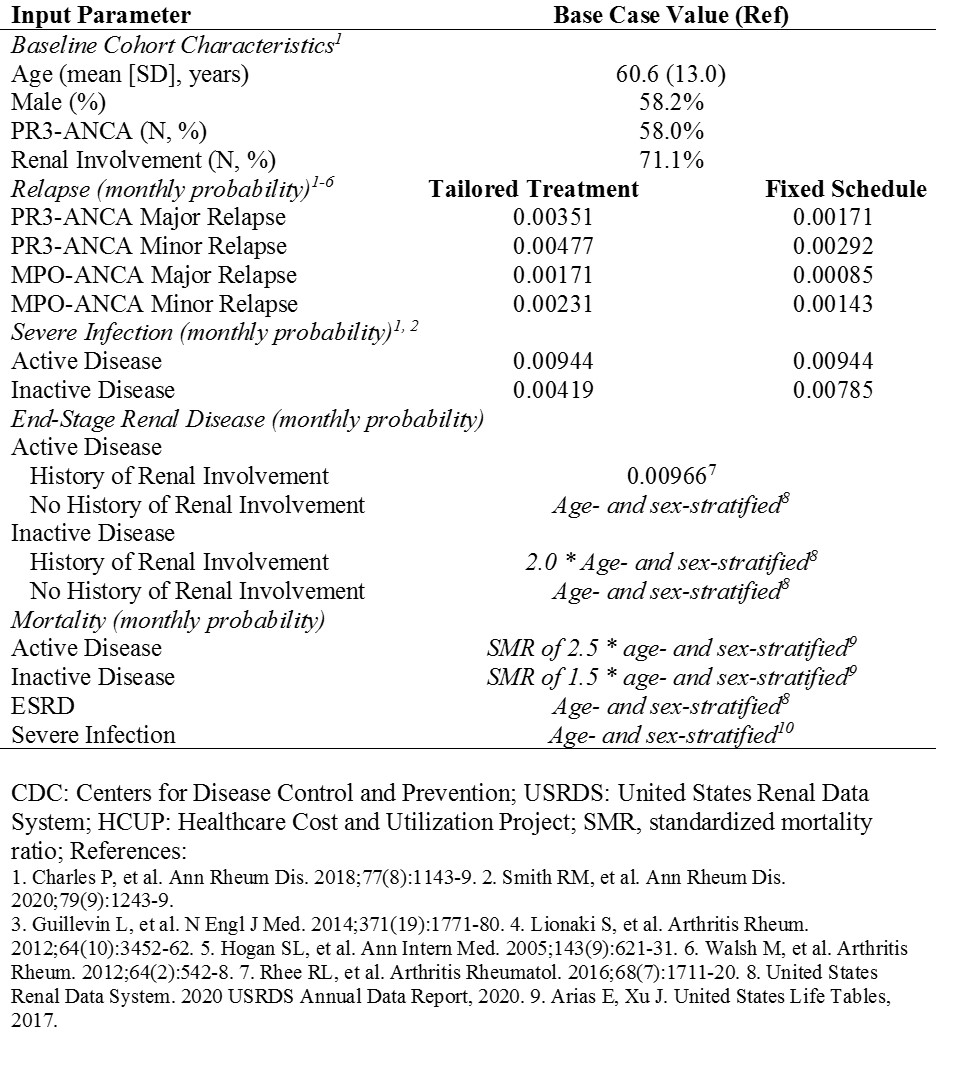
Methods: We developed a state-transition, microsimulation model of people with AAV (TreeAge Pro Healthcare 2020). Figure 1 displays the health states; individuals remain in or transition between health states monthly. At model start, individuals draw for demographic and disease-specific characteristics and then transition between the active AAV or inactive states (i.e., relapse/remission, stratified by major/minor severity) and are at risk for severe infection, ESRD, or death. Transition rates are stratified by demographic and disease-specific characteristics. Table 1 shows model input parameters. The distribution of baseline demographics (i.e., age, sex, ANCA type, renal involvement) are from the MAINRITSAN 2 trial, which compared fixed-schedule rituximab (RTX) to a tailored approach based on rising ANCA or B cell titers for remission maintenance. We obtained probabilities of relapse, severe infection, and ESRD from MAINRITSAN 2, and other clinical trials, cohort studies, and national registries. We estimated mortality based on disease-specific features and background age- and sex-adjusted rates derived from US life tables. We then used the AAV model to project outcomes with tailored vs fixed-schedule RTX among people in remission at baseline over 28 months, as studied in MAINRITSAN 2. We performed face validity assessment with experts in the field. We used mean average percent error (MAPE) to assess how the model-projected outcomes compared to those observed in MAINRITSAN 2.
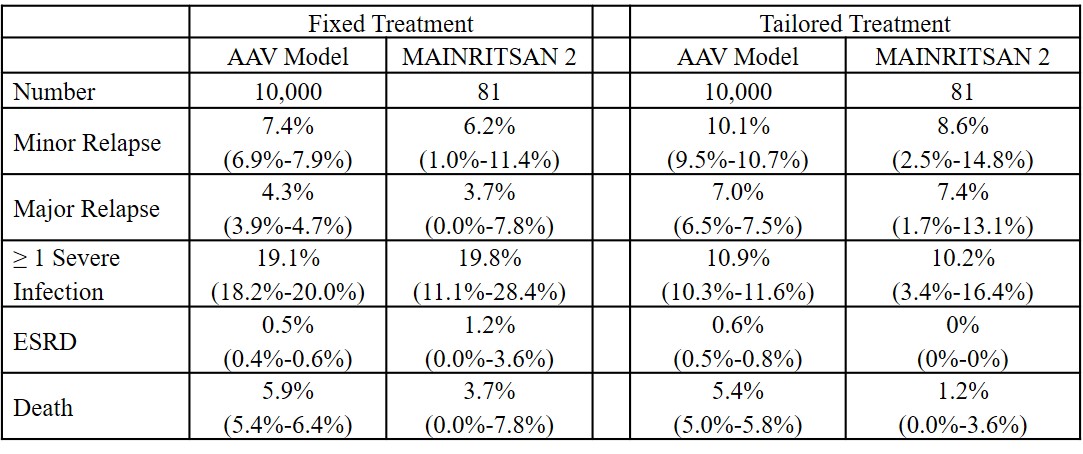
Results: Over 28 months, the AAV model projected fewer minor and major relapses with fixed vs tailored RTX (7.4% [95% CI 6.9%-7.9%] vs 10.1% [9.5%-10.7%] and 4.3% [3.9% vs 4.7%] vs 7.0% [6.5%-7.5%]). More patients had at least one severe infection in the fixed vs tailored group (19.1% [18.2%-20.0%] vs 10.9% [10.3%-11.6%]). ESRD was uncommon in both groups (0.5% [0.4%-0.6%] vs 0.6% [0.5%-0.8%]). The mean survival was the same in both strategies (27.0 ± 3.9 vs 27.0 ± 3.8 months). Compared to the MAINRITSAN 2 trials results (Table 2), the projected rates of relapse, severe infection, ESRD, and death in the AAV model were similar (MAPE, 1.5%, range 0.4%-4.3%). Limitations include that we made assumptions regarding differences in mortality rates during active vs inactive disease states.
Conclusion: We established the face validity and internal validation of a novel AAV state-transition model that projects key outcomes, including minor and major relapse, severe infection, ESRD, and death. This model can be adapted to compare other strategies (e.g., azathioprine) and incorporate other health states (e.g., steroid toxicity), quality of life, and costs.
To cite this abstract in AMA style:
Wallace Z, Stone J, Choi H, Hyle E. Development and Validation of a Simulation Model for Maintenance Treatment in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-simulation-model-for-maintenance-treatment-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-simulation-model-for-maintenance-treatment-in-anca-associated-vasculitis/