Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Giant cell arteritis (GCA) causes inflammation of the blood vessels of the head and neck and can cause visual loss and large vessel vasculitis. Patients suffer severe headaches, pain around the scalp and jaw and musculoskeletal symptoms. Treatment includes high dose glucocorticoids, to control symptoms and protect sight.
The aim of this study was to produce a validated disease-specific PROM for patients with GCA, to capture the impact of GCA and its treatment on health-related quality of life
Methods: This was a large cross-sectional survey including patients with clinician-confirmed GCA (cranial, visual loss and large vessel vasculitis) from the UK. Participants with a diagnosis date within the last three years OR flare of disease within the last year were recruited, to enrich the sample of participants with active disease. An underpinning qualitative study and cognitive testing developed 40 candidate items each with a 5-point Likert scale [1]. Patients completed this 40-item draft GCA-PROM alongside EQ5D-5L, CAT-PRO5 and self-report disease activity and steroid usage.
The statistical analysis aimed at determining the construct validity with Rasch models and factor analysis performed iteratively. Items were fitted to the Rasch model to determine its construct validity, reliability, unidimensionality and statistical sufficiency of the total score from the scale. Factor analysis was used to establishing factor structure. Item reduction decisions were be based on clinical importance, lack of fit to the Rasch model, and redundancy.
Further evidence of validity was tested by comparing the scores of the newly validated GCA-PROM (i) in participants who self-identify as having ‘active disease’ versus patients ‘in remission’ (known groups validity) (ii) with scores derived from EQ5D-5L and CAT-PRO5 (convergent validity).
Results: The validation sample comprised 426 patients, with a mean (SD) age of 74.2 (7.2), 327 (76%) had cranial GCA and 285 (67%) were female.
The initial analysis of the 40 items, resulted in a lack of fit to the Rasch model and led to the deletion of 10 items. Of the remaining items, 22/30 displayed disordered thresholds necessitating collapsing two categories (very mild and mild) resulting into a 4-response category structure which improved the thresholds.
Four factors (domains) were identified: Acute symptoms (8 items), Activities of daily living (7 items), Psychological (7 items) and participation (8 items). The four domains were analysed as ‘super-items’ and shown to fit the Rasch model. Table 1 presents item parameters for each of the 4 domains (non-significant Chi-Square probabilities indicate fit to the Rasch model).
The overall scale had an adequate fit to the Rasch model: X2 = 25.219, DF=24, p=0.394 including reliability PSI=0.828. The raw-to-linear transformation scale was calibrated to enable parametric analyses if desired.
Each domain was shown to have known-groups validity and correlation with EQ5D-5L and CAT-PRO5 (Rs) ranging between 0.4.42 and 0.778 (Table 2).
Conclusion: The final 30-item GCA-PROM with 4 domains is a robust measure of health-related quality of life in people with GCA. Further work will explore its use in clinical trials and practice.
 Table 1: Fit statistics for each of the four domains GCA-PROM
Table 1: Fit statistics for each of the four domains GCA-PROM
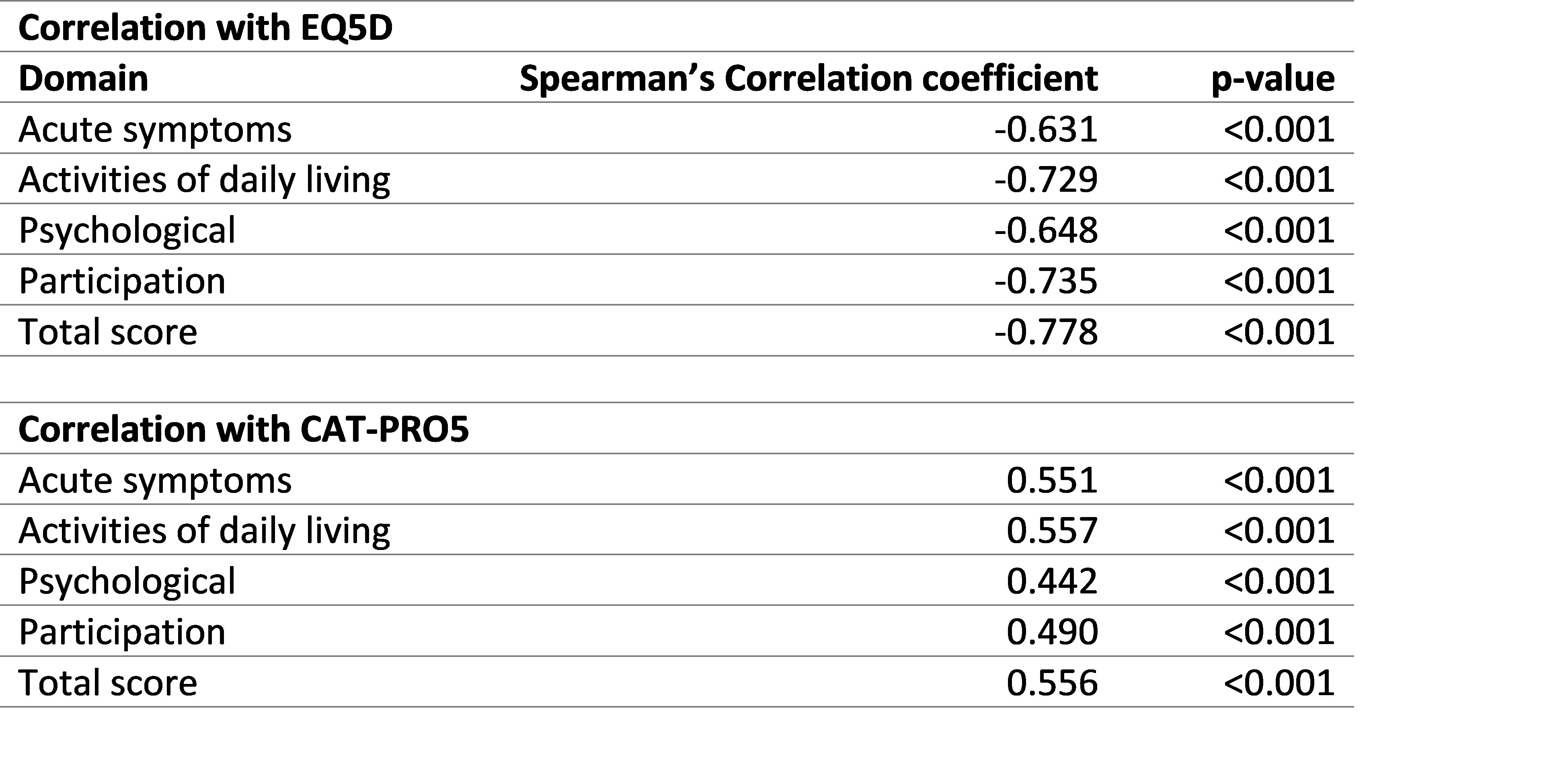 Table 2: Convergent validity of the four domains with EQ5D-5L and CATPRO5
Table 2: Convergent validity of the four domains with EQ5D-5L and CATPRO5
To cite this abstract in AMA style:
Ndosi M, Almeida C, Dawson J, Dures E, Greenwood R, Guly C, Mackie S, Bromhead A, Stern S, Robson J. Development and Validation of a Patient Reported Outcome Measure for Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-patient-reported-outcome-measure-for-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-patient-reported-outcome-measure-for-giant-cell-arteritis/
