Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) features unpredictable disease activity fluctuations, making flare hard to detect and significantly impairing quality of life. This highlights the need for improved patient-reported outcomes (PROs) and the development of patient-centered tools that allow individuals to monitor their disease activity and burden, empowering them to better manage their condition.
This work aims to develop and evaluate the characteristics of a self-report questionnaire (LUPIN) to assess SL disease activity and lupus-relevant PROs.
Methods: Patient representatives from a national lupus association (AFL+) and lupus experts developed a self- report questionnaire which included several domains of SL perceived activity (Fig. 1) and SF36. Prior to a medical consultation, the patient filled the questionnaire which was sealed. The physician then blindly evaluated the patient and documented their characteristics along with a physician global assessment (PhGA) and a disease activity index (SLEDAI-2K). The questionnaires were distributed to 34 centers in metropolitan France and its overseas territories. Correlations between patient response in individual domains and physician assessment were evaluated using Spearman’s regression and Student’s test was used to determine causes of discordance between PROs and clinician assessment.
Results: Questionnaires from 444 patients were analyzed, with a mean age of 45 [44;47] years including 85% women. Average SLE duration was 14 [13;15] years. Most patients had history of articular and cutaneous involvements (86% and 72%), 35% had lupus nephritis and 29% scored a SLEDAI ≥ 4, with an average score of 3.4 [3.0;3.9].
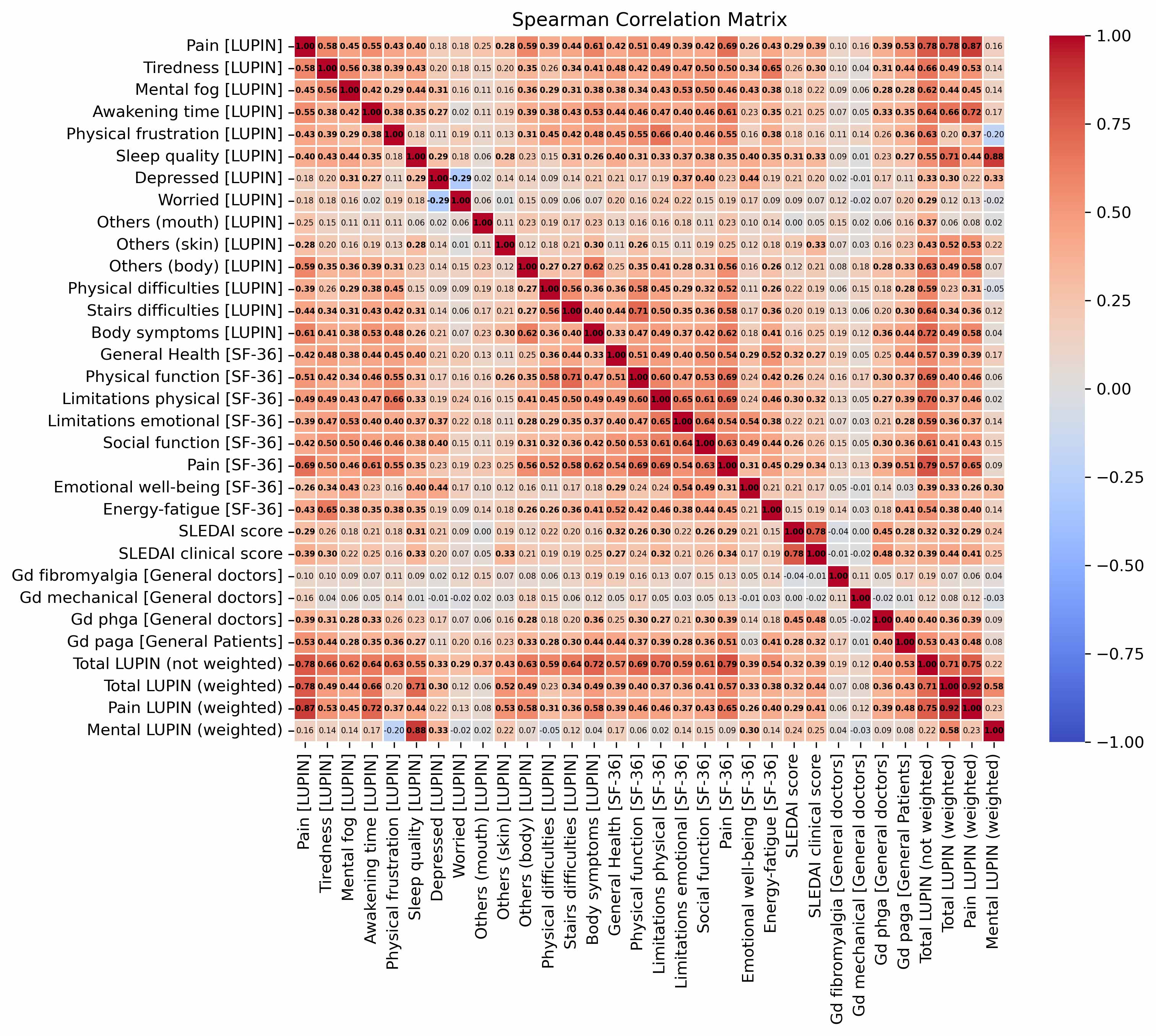
PhGA correlated moderately with SLEDAI and clinical SLEDAI (r = 0.45 and 0.48). As expected, there was a weak albeit statistically significant correlation between LUPIN components and SLEDAI, cSLEDAI or PhGA (r < 0.39 for all). However, there were strong correlations between several LUPIN components and SF36 domains (r = 0.69 for pain evaluation; 0.66 for physical function; 0.65 for fatigue). A LUPIN global composite score and SF36 physical and pain domains correlate also well: 0.69 < r < 0.79 (p < 0.0001 for all correlations cited above; Fig. 2).
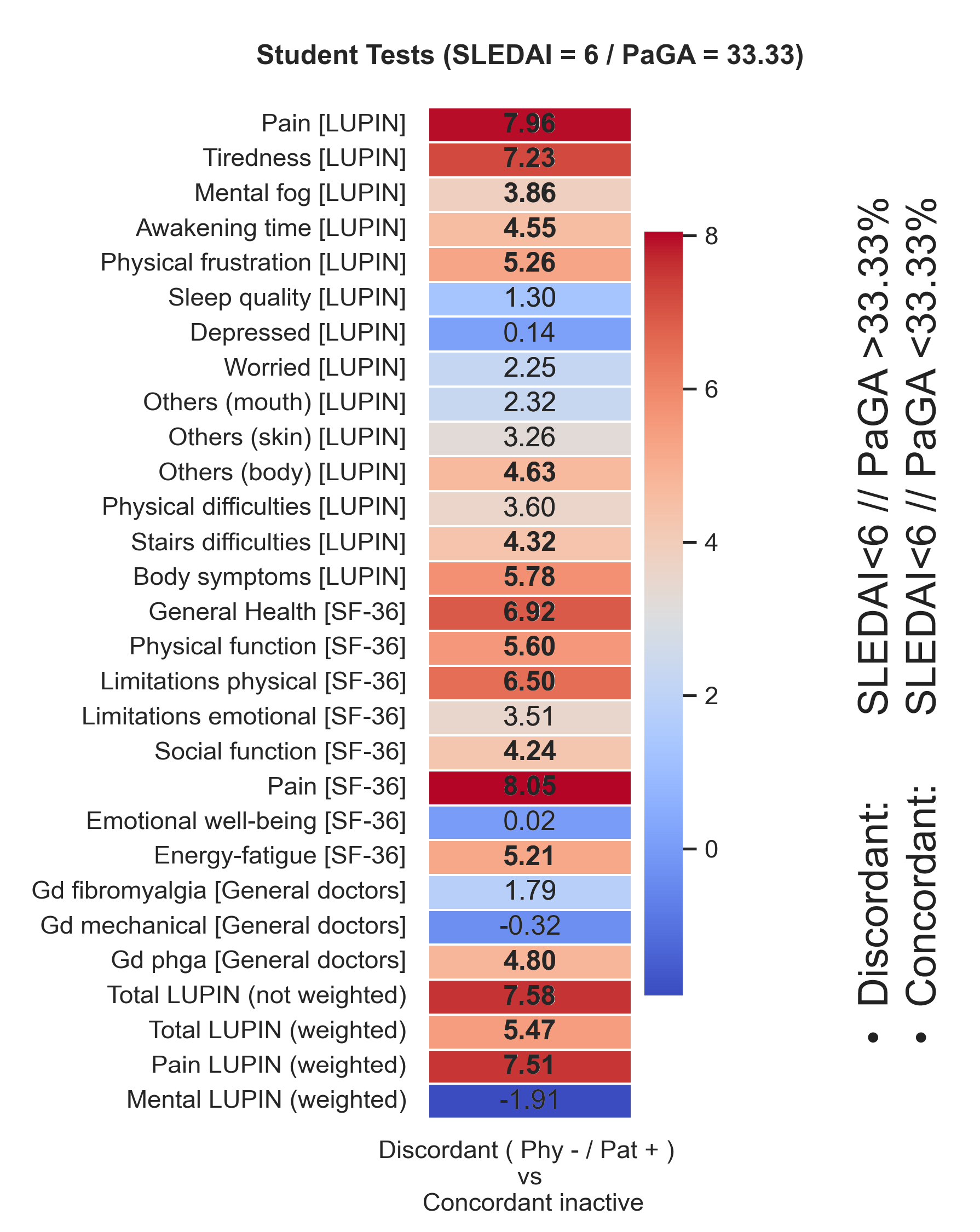
After classifying patients into 3 groups based on physician-patient disease activity agreement (Concordant active, Concordant inactive, Discordant), we found that the most significant differences between subgroups are in patient’s pain and fatigue perception, unrelated to physician’s suspicion of central pain sensitization or mechanical pain (Fig. 3).
Conclusion: Several LUPIN components have a significant correlation with SF36 domains, making LUPIN a patient-friendly tool for evaluating PROs. As expected, a simple classic linear correlation in this study, where 2/3 of the population had low activity (SLEDAI < 4), showed weak correlations between LUPIN and physician-assessed disease activity. A complex relationship between SLEDAI & LUPIN is being tested with AI / deep learning to predict SLEDAI more accurately. Monitoring LUPIN prospectively could offer a more sensitive method for detecting fluctuations in disease activity. This approach is being tested in an ongoing smartphone-based study.
To cite this abstract in AMA style:
Scherlinger M, Kleinmann J, Folliasson A, Riviere M, Rybak R, Malivoir S, Viallard J, Lazaro E, Richez C, Machelart I, Magy-Bertrand N, Gorse A, Blaison G, Campagne J, Dervieux B, Moulinet T, Jaussaud R, Roblot P, Puyade M, Servettaz A, Orquevaux P, le Scanff J, WENDLING D, Andre M, Trefond L, SMETS P, Baillet N, Deligny C, Mariette X, HOT A, David E, Perard L, Jean E, Permal S, WAHL D, Agard C, Chasset F, Hervier B, Ronan P, Martin M, Lebourg L, Renou F, Raffray L, Diot E, Fermont C, Martin T, Korganow A, Gottenberg J, Sibilia J, Amoura Z. Development and Validation of a Patient-centered Self-evaluation Questionnaire in Systemic Lupus Erythematosus: LUPIN® [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-a-patient-centered-self-evaluation-questionnaire-in-systemic-lupus-erythematosus-lupin/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-a-patient-centered-self-evaluation-questionnaire-in-systemic-lupus-erythematosus-lupin/