Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis (OA) is a debilitating disease affecting ~54% of US adults 75 and older. Toll-like receptor 4 (TLR4), and its co-receptor CD14, have been implicated in disease severity and progression. CD14 sensitizes TLR4 to low concentrations of its ligands, including ligand lipopolysaccharide (LPS). Osteoblasts (OB) from the mesenchymal cell (MSC) lineage are not known to express significant levels of CD14 but do express TLR4. As a result, the role of CD14 on OB differentiation has not been fully elucidated. However, the response of OB to LPS has been reported to be multi-faceted and dependent on LPS concentration; the effect may be stimulatory at low concentrations and inhibitory at high concentrations. With this, we aimed to identify the effect of CD14 deficiency on bone remodeling in a murine model of OA in vivo and characterize the effect of low-level, OA-relevant LPS stimulation in vitro.
Methods: We first performed destabilization of the medial meniscus (DMM) or sham surgery on 4-5 C57BL/6 (WT) and CD14KO male mice at 12 wks of age. After 2-, 4-, and 8-wks post-surgery, the knees were processed and stained for alkaline phosphatase (ALP) using ELF 97 fluorescent substrate followed by TOPRO3 nuclear counterstaining. Area of ALP+ staining in the medial tibial subchondral area was quantified and normalized to overall trabecular area. In vitro, MSCs were harvested from 5-7 WT and CD14KO male mice at 12 wks of age. Upon confluency, cells were passaged and cultured for 14d with osteogenic differentiation media (ODMMEM + 10% FBS + ascorbic acid + -glycerophosphate) with or without 1 ng/mL of LPS. After 14d, the cells were stained for ALP and imaged at 10x. Percent area of ALP (+) staining was quantified via Cell Profiler.
Results: Fig. 1A-F depicts representative sections from naïve, sham, and DMM-operated WT and CD14KO mice stained for ALP. At 2wks ALP activity seemed to be increased in the surgical groups of both strains,but no significance differences were observed (Fig. 1G) At 4wks, the area of ALP was increased in both the WT and CD14KO DMM groups compared to either naïve (WT) or sham (CD14KO) controls (Fig 1H). By 8 wks, there was no difference between WT DMM and WT control groups (Fig 1I). However, ALP+ area remained elevated in the CD14KO DMM group compared to both sham controls, and the WT DMM group (Fig 1I). In vitro staining for ALP revealed no significant difference between strains, with or without LPS both qualitatively (Fig. 2A-D) and quantitatively (Fig 2E).
Conclusion: In conclusion, our findings suggest OB density is increased in the remodeling subchondral bone post-DMM injury, and the response of WT and CD14KO mice is similar up to 4 wks. However, by 8 wks, ALP activity in WT DMM mice decreased back to baseline, but remained elevated in the DMM-injured CD14KO mice, suggesting prolonged or delayed OB-genesis in response to DMM in CD14KO mice. Thus far, our in vitro experiments did not reveal an inherent defect in osteoblast differentiation evaluated by ALP staining between strains, either in the presence or absence of low concentrations of LPS. Future experiments will more fully characterize the effects of LPS dosage and of related gene expression to determine whether OB differentiation or activity is altered on WT and CD14KO mice.
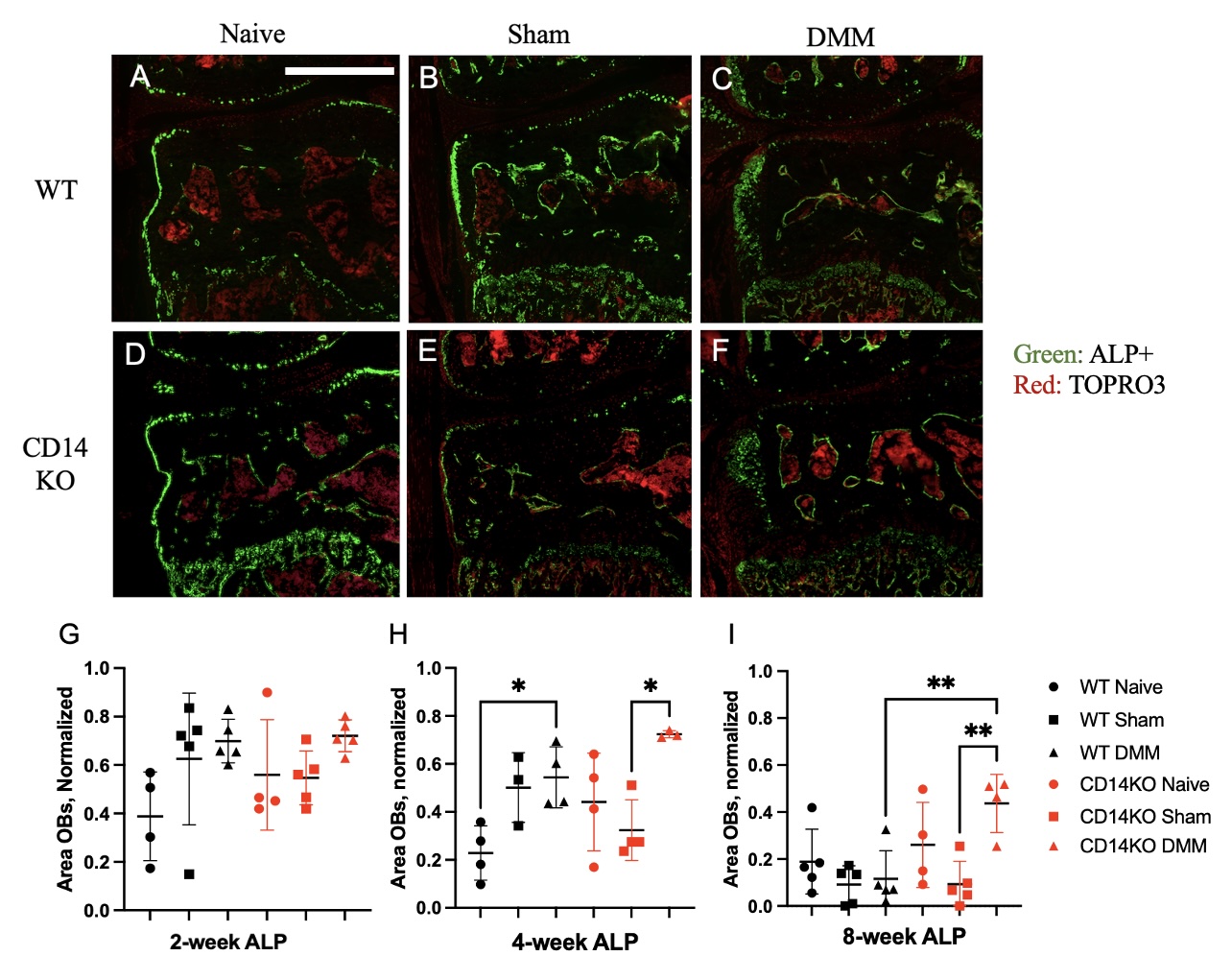 Figure 1: (A-F) Representative images of in vivo ALP activity 4-wks post-surgery. (A) WT Naïve, (B) WT Sham, (C) WT DMM, (D) CD14KO Naïve, (E) CD14KO Sham, (F) CD14KO DMM. (G, H, I) 2, 4, and 8-wk quantification of OB area in the medial subchondral bone region normalized to trabecular area. *p < 0.05, scale bar = 500 µm.
Figure 1: (A-F) Representative images of in vivo ALP activity 4-wks post-surgery. (A) WT Naïve, (B) WT Sham, (C) WT DMM, (D) CD14KO Naïve, (E) CD14KO Sham, (F) CD14KO DMM. (G, H, I) 2, 4, and 8-wk quantification of OB area in the medial subchondral bone region normalized to trabecular area. *p < 0.05, scale bar = 500 µm.
.jpg) Figure 2: (A-D) Representative images of in vitro ALP activity after 14 days in cell culture imaged in the Cy5 fluorescent channel at 10x. (A) WT MSCs cultured with ODM. (B) CD14KO MSCs cultured with ODM. (C) WT MSCs treated with ODM + 1 ng/mL of LPS. (D) CD14KO MSCs treated with ODM + LPS. (E) Quantification of % area of ALP normalized by total area.
Figure 2: (A-D) Representative images of in vitro ALP activity after 14 days in cell culture imaged in the Cy5 fluorescent channel at 10x. (A) WT MSCs cultured with ODM. (B) CD14KO MSCs cultured with ODM. (C) WT MSCs treated with ODM + 1 ng/mL of LPS. (D) CD14KO MSCs treated with ODM + LPS. (E) Quantification of % area of ALP normalized by total area.
To cite this abstract in AMA style:
Sharp K, Murphy L, Nguyen V, Hu B, Scanzello C. Determining the Effect of CD14 Deficiency on Osteoblastogenesis and Subchondral Bone Remodeling In Mice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/determining-the-effect-of-cd14-deficiency-on-osteoblastogenesis-and-subchondral-bone-remodeling-in-mice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/determining-the-effect-of-cd14-deficiency-on-osteoblastogenesis-and-subchondral-bone-remodeling-in-mice/
