Session Information
Date: Tuesday, November 14, 2023
Title: (1776–1795) Spondyloarthritis Including Psoriatic Arthritis – Basic Science Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic, progressive autoimmune condition that affects both the skin and joints. It is characterized by increased levels of the proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) (Guttman-Yassky et al., 2011). TNF signals via two receptors, TNFR1 and TNFR2. Recently, our lab discovered that globally deleting the TNFR2 gene, while keeping TNFR1 intact, can reduce psoriasis-like inflammation in mice (Chandrasekharan et al., 2022). Mechanistically, we found that TNFR2 plays a crucial role in the dendritic cell (DC)-dependent production of IL-23 and polarization of TH17 cells. In psoriasis, DCs are known to be involved in capturing self-antigens from abnormal skin cell growth, presenting them to T cells, and initiating an immune response (Kamata and Tada, 2022). The current research sheds light on the critical role of TNFR2 in DC activation and the development of PsA, offering potential avenues for targeted therapeutic therapies. In this study, we are testing if targeting TNFR2 specifically in DCs using conditional knockout mice can alleviate PsA.
Methods: We investigated the impact of DC-specific TNFR2 knockout (DC-TNFR2KO) on skin lesions and joint inflammation in a mouse model of psoriatic arthritis induced by Mannan (Khmaladze et al., 2014). Psoriasis Area and Severity Index (PASI) Score and arthritis severity score calculations were performed on TNFR2fl/fl (control) and DC-TNFR2KO mice (TNFR2fl/fl-CD11cCre). Histological evaluations were performed on ear skin and paw samples using H&E staining and safranin staining, respectively. Histopathological analyses and scoring were conducted in a blinded manner by independent investigators. Additionally, ELISA was used to determine whether DC-dependent cytokine production was altered in DC-TNFR2KO mice in response to Mannan treatment. Statistical analyses were performed with the SPSS version and Prism (GraphPad Software, San Diego, CA).
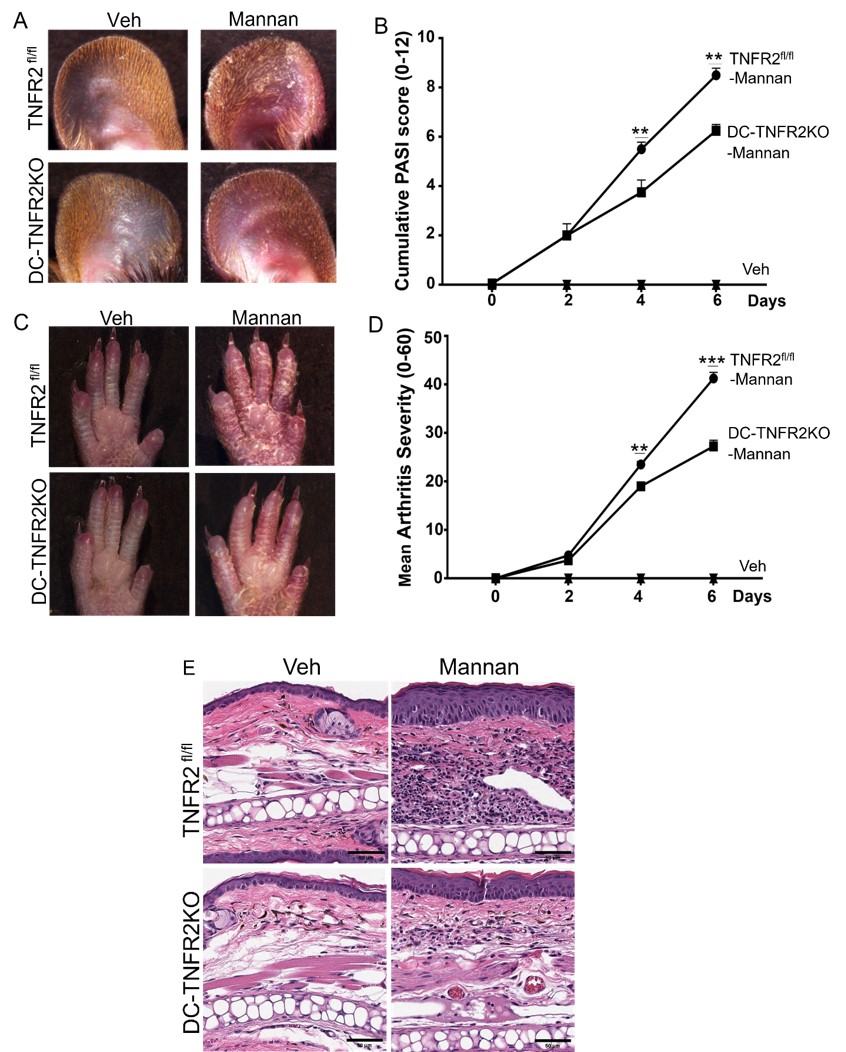
Results: Following Mannan injection TNFR2fl/fl mice developed PsA-like skin lesions and joint inflammation, while DC-TNFR2KO mice exhibited minimal symptoms (Figure 1A-B). The cumulative PASI score was significantly lower in DC-TNFR2KO mice compared to TNFR2fl/fl mice at days 4 and 6 after Mannan injection (p < 0.01, each). The mean arthritis severity score was also lower in DC-TNFR2KO mice (p < 0.01; p < 0.001) (Figure 1 C-D). Histological examination revealed that TNFR2fl/fl mice had significantly increased epidermal thickness (Figure 1E) and leukocyte infiltration compared to DC-TNFR2KO mice after Mannan injection (p < 0.001; p < 0.01). Safranin O staining showed cartilage loss in both TNFR2fl/fl and DC-TNFR2KO mice, however, it was less in TNFR2KO compared to TNFR2fl/fl mice (p< 0.05). Furthermore, we found diminished levels of IL-12, IFN-γ and TNF-α in the serum of DC-TNFR2KO mice compared to TNFR2fl/fl mice, post Mannan treatment (p< 0.0001; p< 0.001; p< 0.01 respectively).
Conclusion: Targeting TNFR2 specifically in dendritic cells can alleviate skin and joint inflammation in a mouse model of PsA. This study provides insights into the mechanistic role of TNFR2 in PsA pathogenesis and may contribute to the development of novel therapies for this debilitating disease.
To cite this abstract in AMA style:
Kaur R, Husni M, Chandrasekharan U, Brambilla R. Dendritic Cell-specific TNFR2 Depletion Reduces Psoriatic Arthritis-like Disease in a Mouse Model [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dendritic-cell-specific-tnfr2-depletion-reduces-psoriatic-arthritis-like-disease-in-a-mouse-model/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dendritic-cell-specific-tnfr2-depletion-reduces-psoriatic-arthritis-like-disease-in-a-mouse-model/