Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare inflammatory disorder characterized by chronic necrotizing vasculitis. Conventional therapy (oral glucocorticoids [OGC] ± immunosuppressants), mepolizumab, and rituximab are used to treat EGPA. Benralizumab has a pending approval for EGPA. This study describes baseline demographics, clinical characteristics, and treatment patterns in patients with EGPA treated with anti-interleukin-5 (IL-5) or IL-5 receptor-α (anti-IL-5/Rα) therapy, rituximab or conventional therapy only. In most cases, rituximab and anti-IL-5/Rα were utilized in addition to OGC, especially at the start of treatment.
Methods: A retrospective, observational, cohort study was conducted using electronic health record/health insurance claims data from the TriNetX Linked USA network. Patients selected were aged ≥18 years, with ≥1 insurance claim/medical record with a diagnosis code for EGPA (ICD-10-CM M30.1), and ≥1 prescription claim/medical record for conventional therapy or new use of anti-IL-5/Rα or rituximab, from Oct 1, 2019 to May 31, 2023. It was not possible to determine what percentage of patients met ACR classification criteria for EGPA. Index date was the first dispensing/administration in the study period post-EGPA diagnosis for conventional therapy or date of first use of anti-IL-5/Rα (mepolizumab or benralizumab) or rituximab. Patients treated with anti-IL-5/Rα or rituximab before EGPA diagnosis were excluded from the study. Treatment use was measured during a 12-month baseline, at index, and 6- and 12-months post-index.
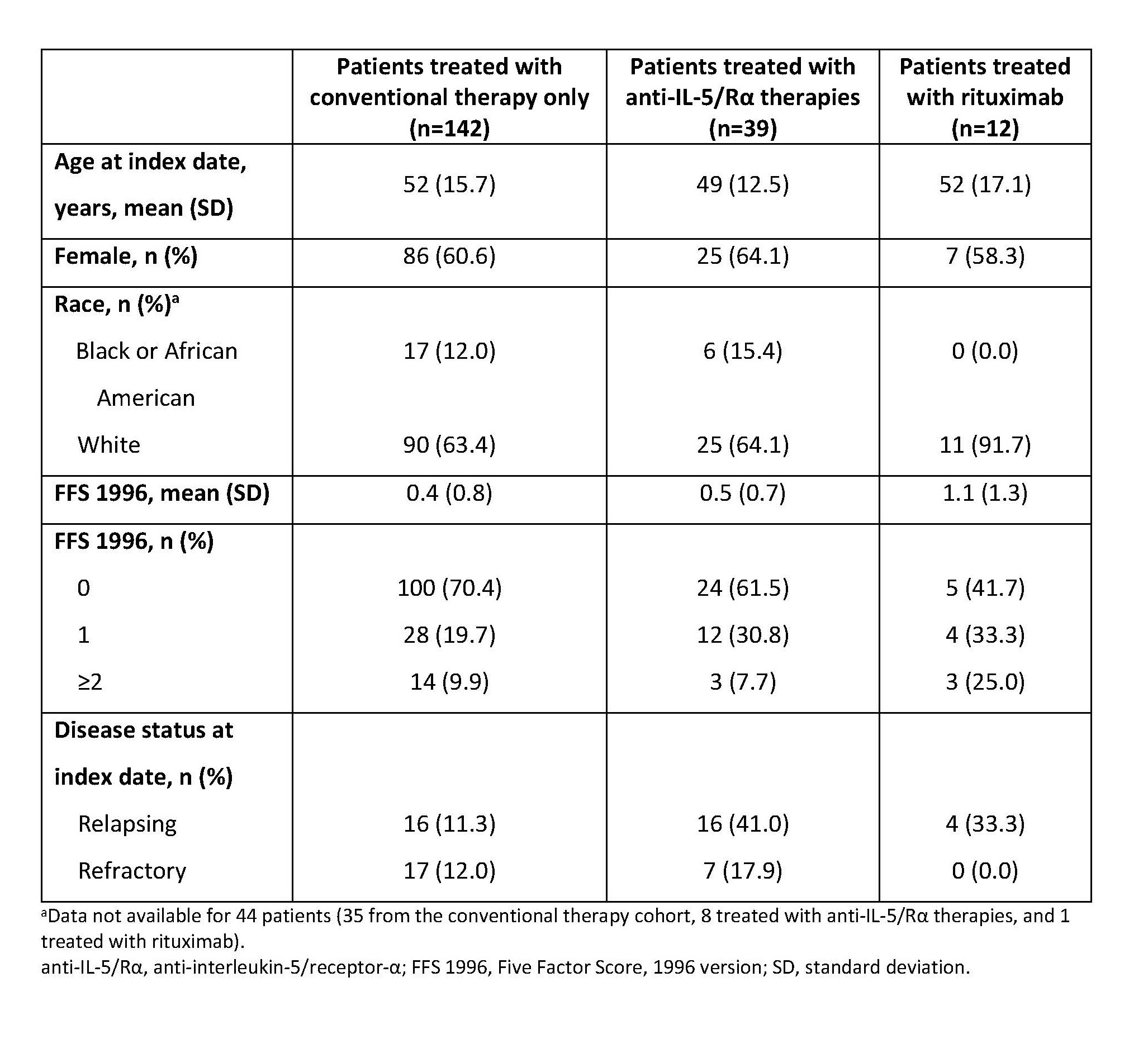
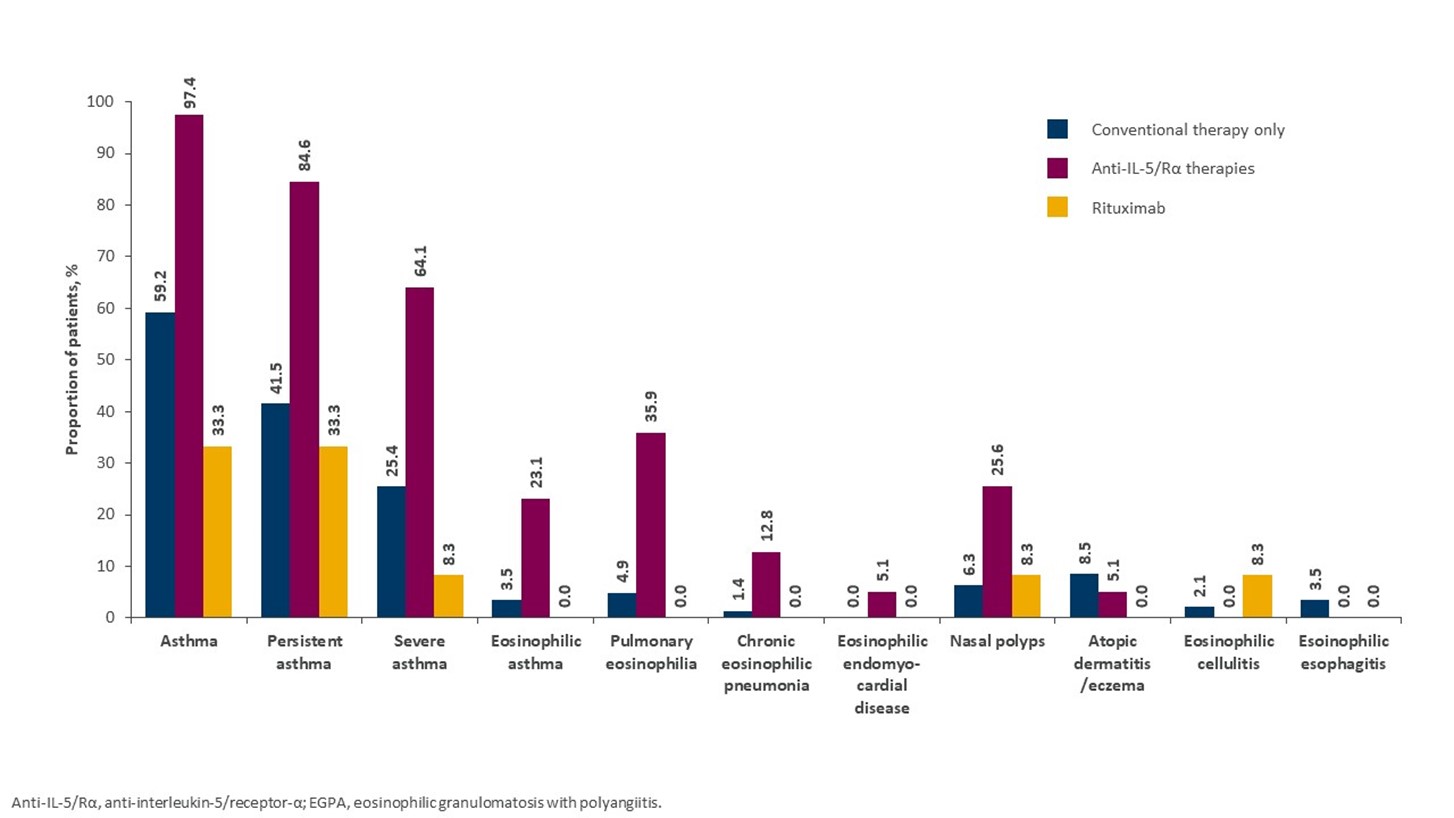
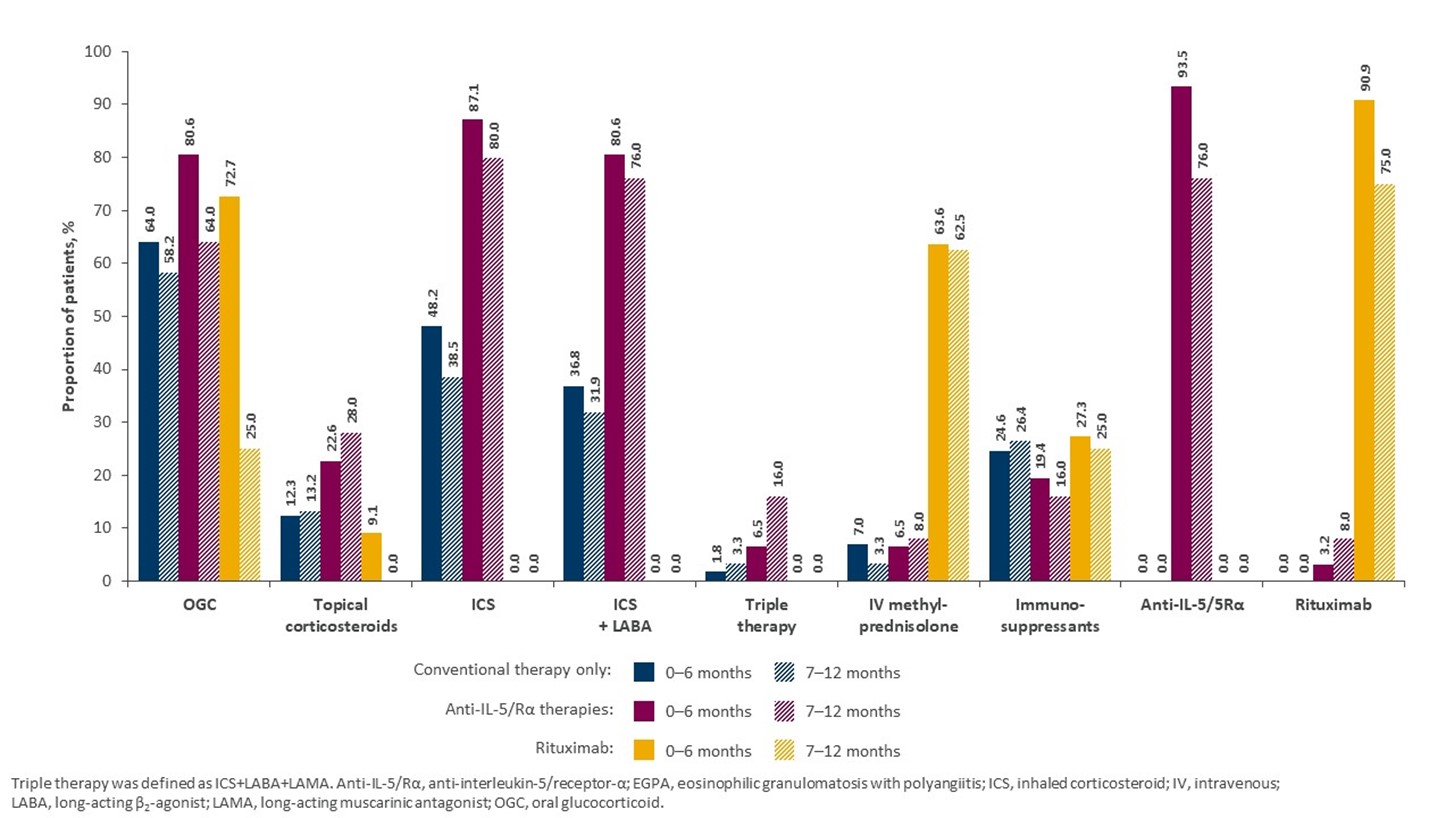
Results: Of 193 eligible patients, 142 received conventional therapy only, 39 received anti-IL-5/Rα, and 12 received rituximab. The mean (standard deviation [SD]) age of patients was 49 (12.5) years in the anti-IL-5/Rα group and 52 (17.1) and 52 (15.7) years in the rituximab and conventional therapy only groups, respectively. Sex ratio was similar across groups, but racial distribution differed (Table 1). Patients with more severe disease, based on Five Factor Score at index date, were more likely to be treated with rituximab, while patients with relapsing or refractory disease were more likely to be treated with anti-IL-5/Rα (Table 1). Patients in the anti-IL-5/Rα group were more likely to have comorbid respiratory (e.g. asthma) or ENT conditions, versus patients treated with conventional therapy only (Figure 1). The proportion of patients treated with OGC post index fell from 80.6% in the first 6 months post index to 64.0% (−16.6%) during months 7–12 post index in patients treated with IL-5/Rα at the index date, whereas a smaller reduction was seen in the conventional therapy only group, 64.0% to 58.2% (−5.8%). Patients treated with rituximab had the greatest reduction in OGC use (−47.7%) (Figure 2).
Conclusion: Patients with EGPA generally had similar demographic characteristics across treatment groups, with the exception of race; EGPA therapies were prescribed in line with disease severity and the nature of comorbid eosinophilic conditions. OGC use decreases were greater with anti-IL-5/Rα than with conventional therapy only, indicating the steroid-sparing potential of eosinophil-targeting biologics in patients with EGPA.
To cite this abstract in AMA style:
Dolin P, Shavit A, Keogh K, Rowell J, Edmonds C, Eudicone J, Moore A, Wilson M, Chan K, Peer T, Chen S. Demographic and Treatment Patterns in Patients with Eosinophilic Granulomatosis with Polyangiitis: A Retrospective Cohort Analysis of US Claims and Clinical Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/demographic-and-treatment-patterns-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-a-retrospective-cohort-analysis-of-us-claims-and-clinical-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/demographic-and-treatment-patterns-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-a-retrospective-cohort-analysis-of-us-claims-and-clinical-data/