Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease with a broad range of clinical manifestations and a high unmet need across patient populations. Real-world data on SLE are scattered across many registries worldwide with heterogeneous data collection. The Lupus Federated Data Network (LupusNet) is an interdisciplinary international initiative that aims to combine and harmonize data from existing SLE registries to create a global, federated network of SLE databases with a larger number of patients, greater data consistency, and the potential to address gaps in understanding of SLE.
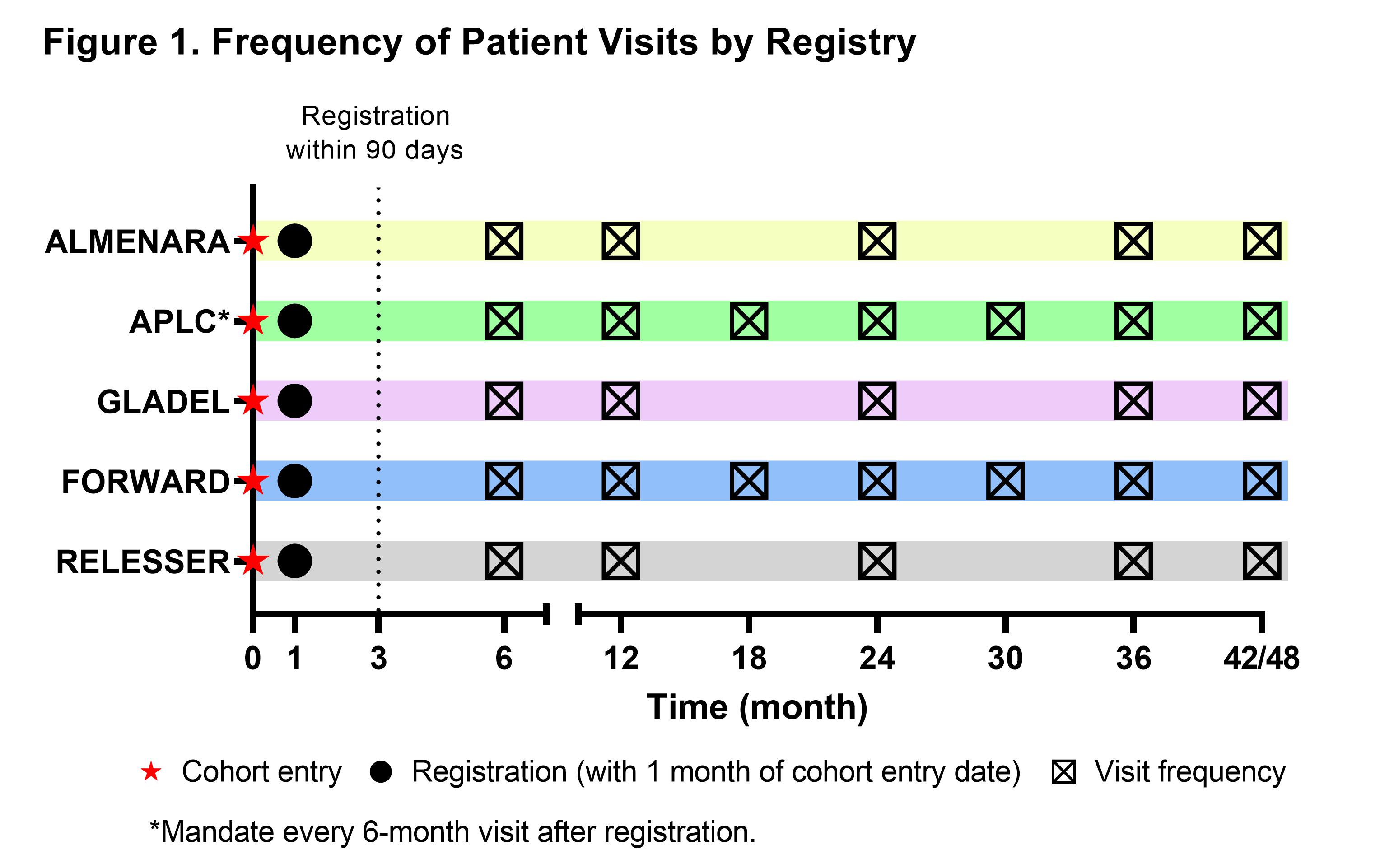
Methods: Data from 5 registries representing >10,000 patients with SLE from 4 regions contributed to LupusNet: APLC (Asia Pacific), RELESSER (Europe), FORWARD (North America), and Almenara and GLADEL (Central and South America). LupusNet uses a federated data network approach and a privacy-by-design method, where the data remains with the respective registries and analysis occurs at the local center, while only aggregated results are shared. Figure 1 illustrates the schedule of visits patients in each registry completed for clinical assessments, including SLEDAI. Demographic and clinical variables (eg, disease activity/severity, clinical events, biopsies/histology, biomarkers, treatment history, comorbidities, medications, and patient-reported outcomes) were mapped and harmonized to the Observational Medical Outcomes Partnership Common Data Model v5.4. This study describes the baseline demographics, characteristics, and disease activity based on the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) in LupusNet during ±90 days of registration.
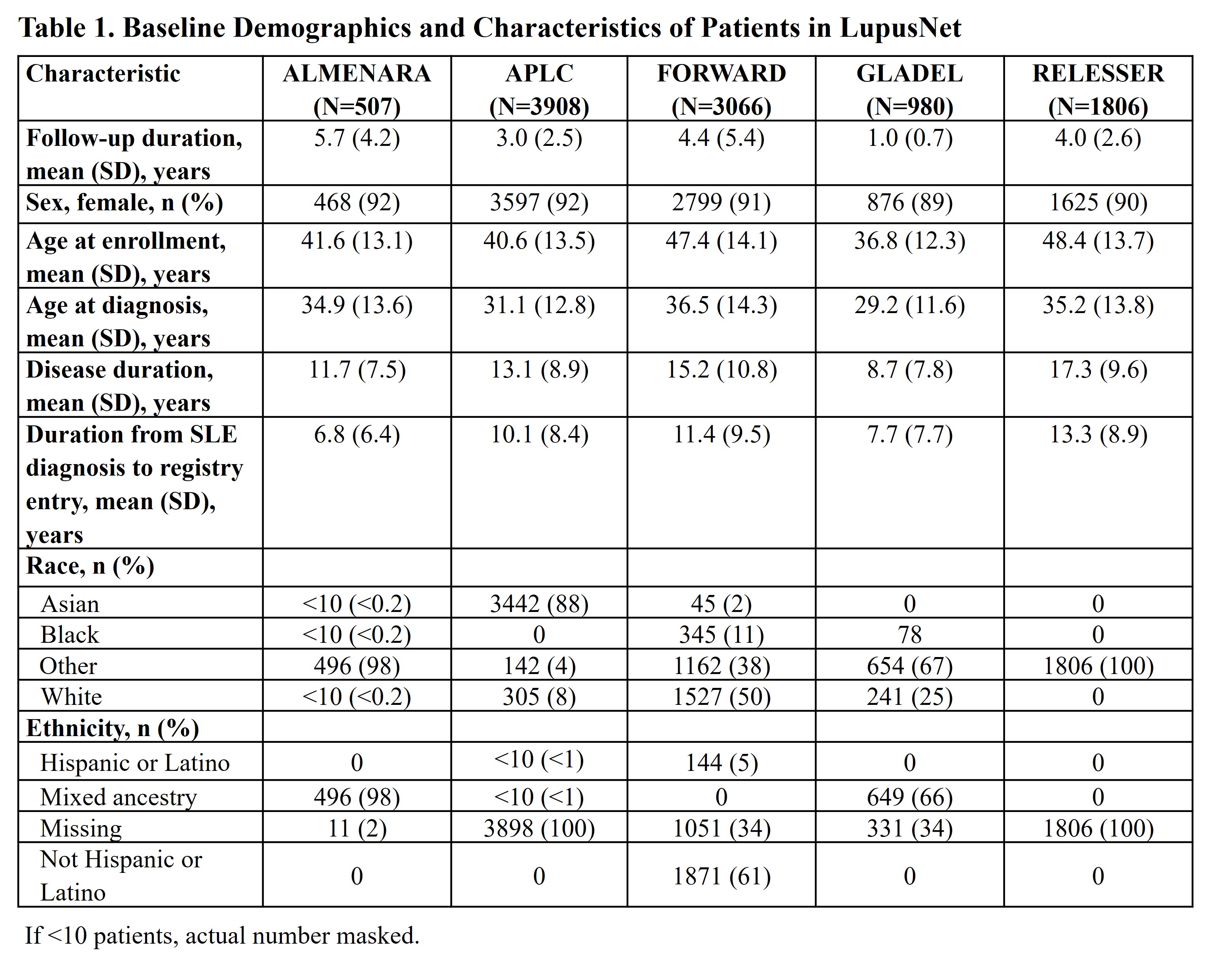
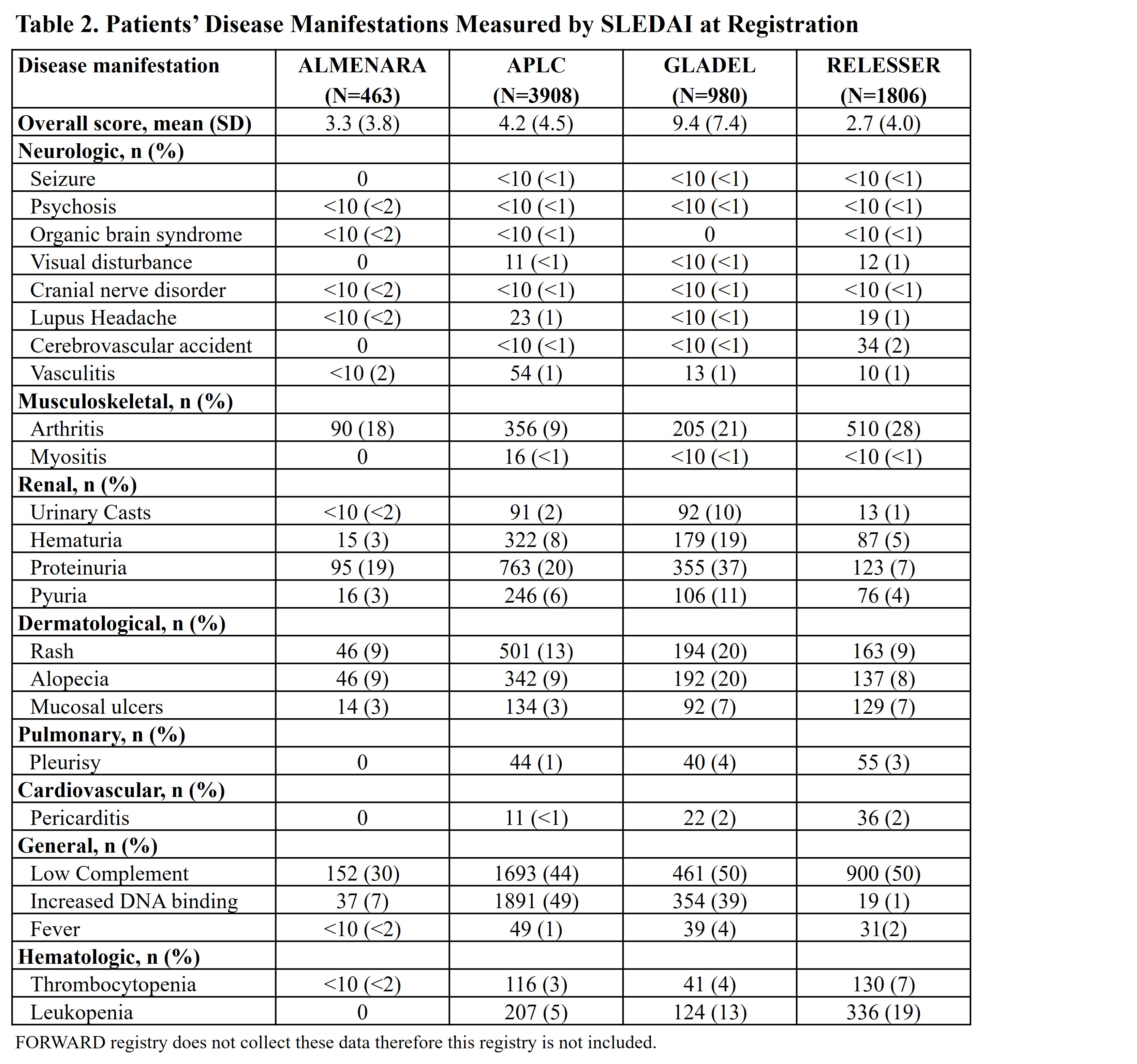
Results: A total of 10,267 patients were included and mapped in LupusNet; of those, 3,908 patients were in Asia Pacific, 1,806 in Europe, 3,066 in North America, and 1,487 in Central and South America. Select baseline demographics and characteristics of patients with SLE are presented (Table 1). Disease activity based on the SLEDAI questionnaire assessed at registration is presented from 4 registries that collected these data (Table 2). Across registries, the majority of patients were female; the duration from SLE diagnosis to registry entry ranged from 5 to 10 years. Heterogeneity and variability in disease manifestations determined from SLEDAI responses were observed across registries, particularly in relation to arthritis, nephritis (ie, proteinuria, pyuria, hematuria, and urinary casts), increased anti-dsDNA antibody, and leukopenia.
Conclusion: Mapping patient characteristics from LupusNet allows researchers to analyze a larger population of patients with SLE across different geographical regions. These findings demonstrate a high degree of variability in disease activity measured by SLEDAI across registries, likely due to their differences in recruitment strategy, treatment strategy/access, healthcare system, and race/ethnicity. Compared to individual registries, this network of collective SLE databases allows further study to better understand disease heterogeneity, patient populations, and treatment patterns with the goal of improving outcomes for patients with SLE across the globe.
To cite this abstract in AMA style:
Zazzetti F, Sbarigia U, Giovanini W, Blacketer C, van Speybroeck M, Simon T, Li G, Karyekar C, Sonmez R, Ugarte-Gil M, Gamboa-Cardenas R, Pimentel-Quiroz V, Pons-Estel G, Pons-Estel B, Quintana R, Saurit V, MONTICIELO O, Corrales K, Esquivel-Valerio J, Rebella M, Pisoni C, Ribeiro F, Núñez-Álvarez C, Michaud K, Katz P, Kandane-Rathnayake R, Morand E, Louthrenoo W, Chen Y, Cho J, Hamijoyo L, Luo S, Wu Y, Navarra S, Sockalingam S, Harigai M, Zhang Z, Basnayake B, Chan M, Takeuchi T, Bae S, Goldblatt F, O'Neill S, Ng K, Poh Y, Tugnet N, Kumar S, Tee M, Tanaka Y, Lau C, Hoi A, Nikpour M, Sapsford M, Rúa-Figueroa Í, Pego-Reigosa J, Galindo-Izquierdo M, Calvo-Alén J, Fernández-Nebro A, Menor Almagro R, Lavie F. Demographic and Clinical Characteristics of Patients with SLE Across 5 Registries – the LupusNet Federated Data Network [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/demographic-and-clinical-characteristics-of-patients-with-sle-across-5-registries-the-lupusnet-federated-data-network/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/demographic-and-clinical-characteristics-of-patients-with-sle-across-5-registries-the-lupusnet-federated-data-network/