Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical trials in early diffuse cutaneous systemic sclerosis (SSc) have historically used the modified Rodnan skin score (mRSS) as the primary outcome measure. These trials have persistently been unable to show any significant improvement in the mRSS between placebo and comparison groups. What has been demonstrated in many trials are declines in the mRSS in both groups; suggesting regression to the mean occurring in both the treatment and the comparison group. We wanted to assess how the definition of disease onset (first SSc manifestation versus first non-Raynaud manifestation), and how varying lengths of disease duration at trial entry as an inclusion criteria functioned with respect to mRSS trajectory. Our objective was to optimize trial inclusion criteria.
Methods: We used a prospective, observational cohort from a large US university, tertiary-care referral Scleroderma Center cohort. We identified early diffuse SSc patients first seen between 1980 and 2015. All had < 3 years from first SSc (n=481) or first non-Raynaud manifestation (n=514) and ≥3 mRSS scores during follow-up. We used descriptive, time to event and group-based trajectory analyses to model: 1) the two different definitions of disease onset (first SSc and first non-Raynaud manifestation), and 2) varying lengths of disease duration as inclusion criteria for clinical trials. Disease duration definitions assessed included < 12 months, < 15 months, < 18 months, < 24 months and < 36 months using both definitions of disease onset.
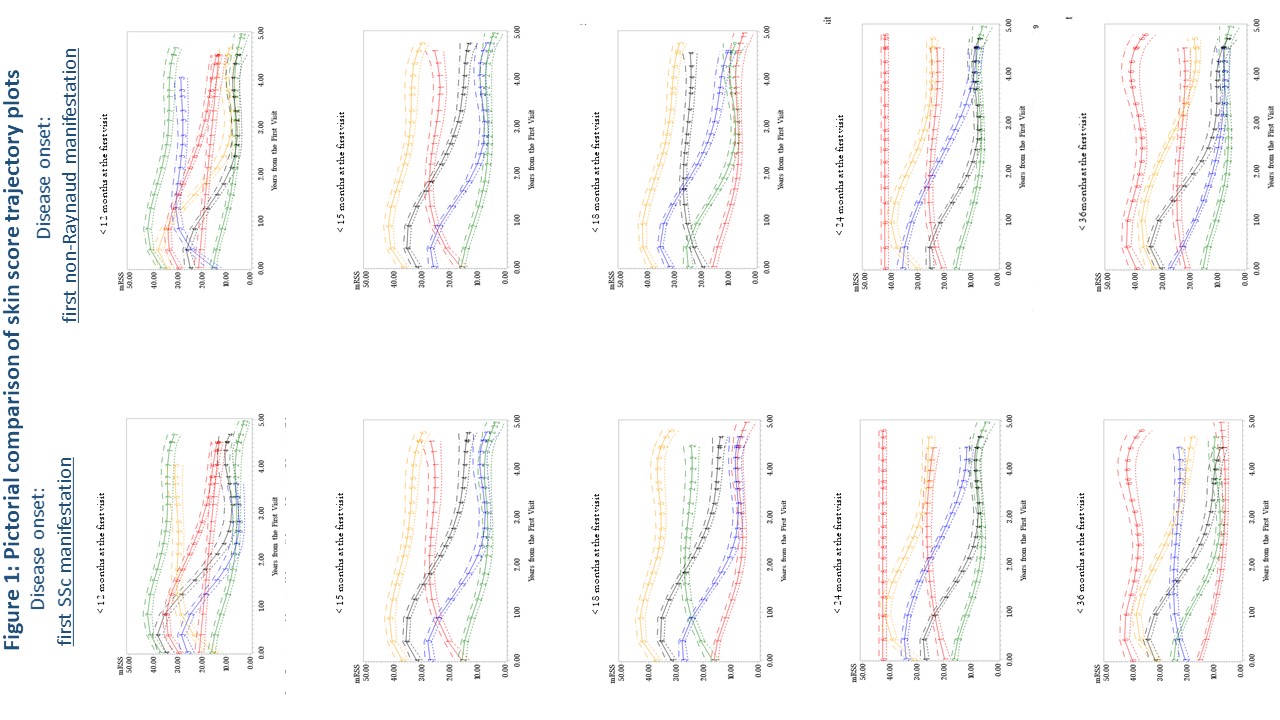
Results: Baseline characteristics are in Table 1. There was no appreciable difference in mRSS trajectory patterns between using first SSc manifestation compared to first non-Raynaud manifestation as the definition of disease onset (Figure 1). Compared to other disease durations, < 18 months of disease had >70% of patients fitting into trajectories with worsening cutaneous disease over the first six months of follow-up. Longer disease durations demonstrated that the majority of patients had trajectories showing an improvement in mRSS (regression to the mean) over six months.
Conclusion: Regardless of whether the first SSc or first non-Raynaud manifestation is used to define disease onset, duration of < 18 months at enrollment is preferable, despite the difficulty in identifying such patients. Longer disease duration criteria more frequently results in regression to the mean of the mRSS score, and may have contributed to prior negative trials. Given the weight of the mRSS in the combined response index (CRISS), this analysis likely applies to trials using the CRISS as an outcome.
To cite this abstract in AMA style:
Domsic R, Gao S, Laffoon M, Wisniewski S, Zhang Y, Lafyatis R, Steen V, Medsger T. Defining the Optimal Disease Duration of Early Diffuse Systemic Sclerosis for Clinical Trial Design [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/defining-the-optimal-disease-duration-of-early-diffuse-systemic-sclerosis-for-clinical-trial-design/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-the-optimal-disease-duration-of-early-diffuse-systemic-sclerosis-for-clinical-trial-design/