Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Although a link between gut microbiome and autoimmune diseases has been suggested, there is a gap in the understanding of the gut microbiome in ANCA-associated vasculitis (AAV). This study evaluated the gut microbiome in AAV (granulomatosis with polyangiitis (GPA), microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis) compared to healthy controls.
Methods:
Using cross-sectional and longitudinal designs, the gut microbiome was compared among patients with i) newly-diagnosed AAV (active and remission); ii) chronic AAV (active and remission), and iii) healthy controls. Fecal samples were collected using standardized methods and analyzed by sequencing the bacterial 16S rRNA gene (V1-V2 region). Taxa with mean abundance >1% were compared using Wilcoxon rank sum test, correcting for multiple comparisons. Disease severity was assessed with the Birmingham Vasculitis Activity Score for WegenerÕs Granulomatosis (BVAS/WG). Effects of medications were studied using mixed effects models.
Results:
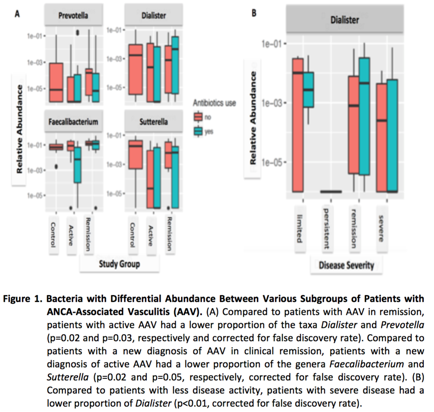
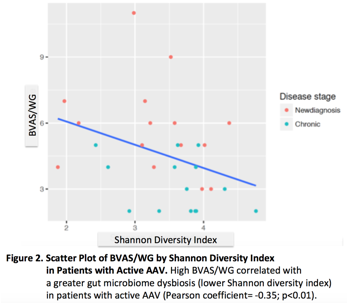
63 fecal samples were studied: 29 active AAV (15 new diagnosis/14 chronic), 20 in remission, and 14 healthy controls. Compared to controls, patients with active AAV had a different microbial composition (p=0.01). There was no statistical difference between the gut microbial composition of controls and patients in remission (p=0.16). The relative abundance of the taxa Dialister and Prevotella were different between active and remission AAV. The relative abundance of the genera Faecalibacterium and Sutterella were different between active and remission newly-diagnosed AAV (Figure 1A). The relative abundance of Dialister was significant in patients with high BVAS/WG compared to patients with low BVAS/WG (p<0.01) (Figure 1B). High BVAS/WG was associated with greater dysbiosis (Figure 2); similar results were found in a multivariate linear model (p=0.02). The gut microbiome in patients with GPA on immunosuppressive agents was similar to controls (p=0.54), whereas the gut microbiome of patients with GPA not on these therapies was significantly different from controls; similar results were found with glucocorticoids and antibiotics use.
Conclusion:
Active AAV is associated with an altered gut microbial composition. Patients in clinical remission have microbial composition similar to healthy controls. Immunosuppressive agents, glucocorticoids, and antibiotics may re-establish a healthy gut microbiome. Severe disease activity is associated with worsening gut dysbiosis suggesting a potential role of gut bacteria in the pathogenesis of AAV.
To cite this abstract in AMA style:
E. Najem C, Lee JJ, Tanes C, Sreih AG, Rhee RL, Geara A, Li H, Bittinger K, D. Lewis J, Merkel PA. Defining the Gut Microbiome in Patients with ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/defining-the-gut-microbiome-in-patients-with-anca-associated-vasculitis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-the-gut-microbiome-in-patients-with-anca-associated-vasculitis/