Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: SpA Including PsA – Diagnosis, Manifestations, & Outcomes I
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: While the Axial Spondyloarthritis Disease Activity Score based on C-reactive protein (ASDAS) is recommended for assessment of disease activity in patients with axial spondyloarthritis (axSpA), the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is still widely used, particularly in settings where inflammatory laboratory markers are unavailable. BASDAI values of < 2, < 4 and >6 have been applied as cut-offs for remission, between low disease activity (LDA) and high disease activity (HDA) and for very high disease activity (VHDA), respectively, although these cut-offs remain unvalidated. We aimed to (i) determine BASDAI cut-offs for disease activity states against predefined external criteria, and (ii) assess the level of agreement between BASDAI and ASDAS disease activity states.
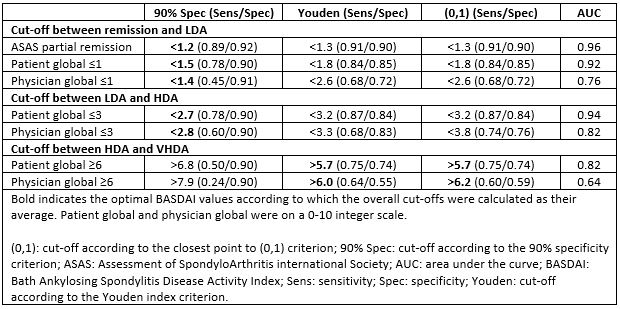
Methods: Prospectively collected real-world data from patients with axSpA initiating a first tumour necrosis factor inhibitor or an interleukin-17A inhibitor from registries participating in the European Spondyloarthritis (EuroSpA) collaboration were analyzed. We applied the same approach as previously used by Machado et al. [1] to define the endorsed ASDAS cut-offs for disease activity states against external criteria, i.e., ASAS partial remission, and patient and physician global assessments. Follow-up data at 6 months were used to select the cut-offs between BASDAI remission and LDA, and between LDA and HDA, while baseline data were used to select the cut-off for VHDA. Receiver operating characteristic analyses were performed to determine the optimal BASDAI cut-offs. The level of agreement between disease activity states based on BASDAI and ASDAS cut-offs was assessed using proportion of discordance and weighted kappa coefficient.
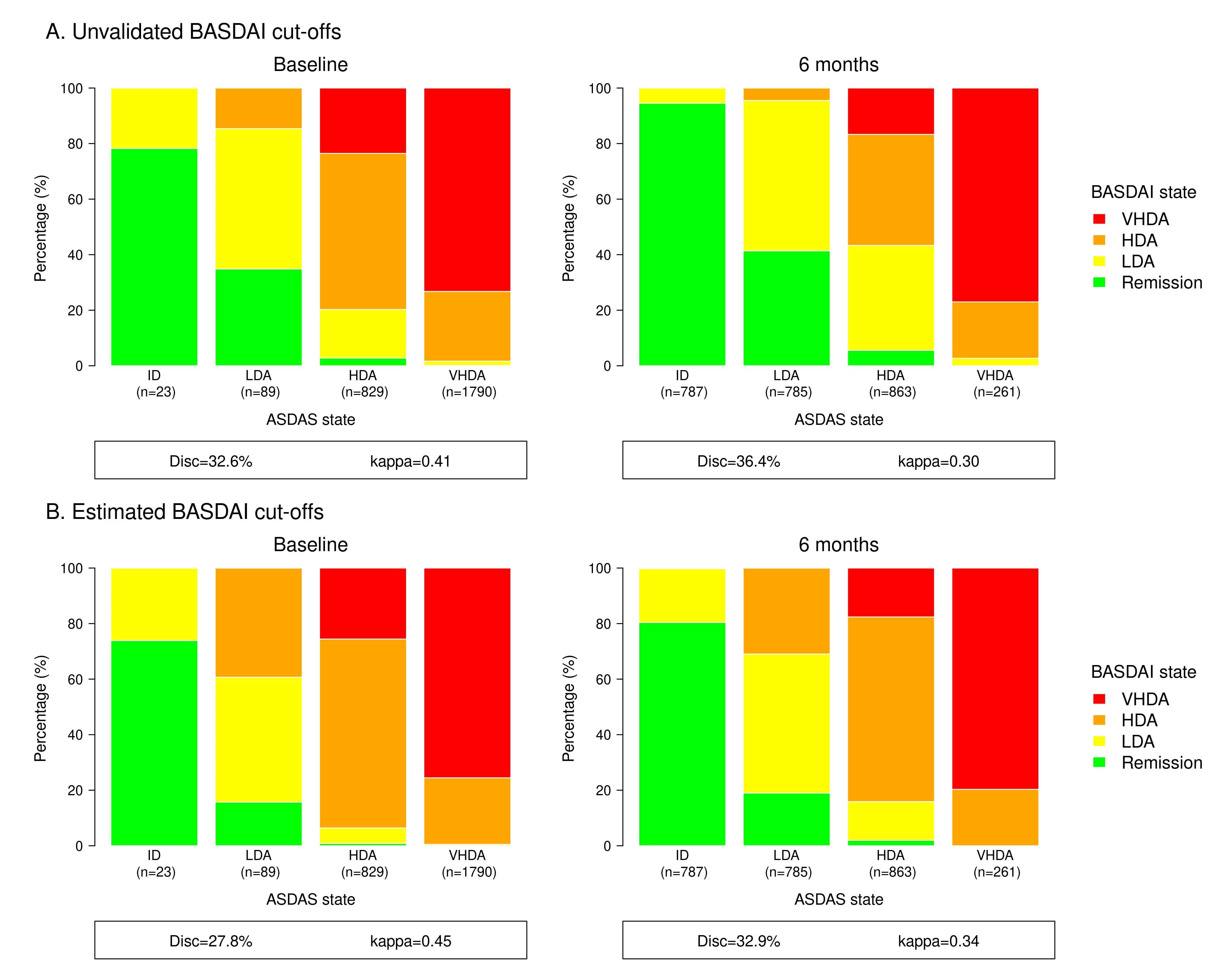
Results: We analyzed data from 2,791 patients from seven European registries with available data on BASDAI and the above-mentioned external criteria. Mean (standard deviation) BASDAI (on a 0-10 scale) and ASDAS were 6.1 (1.9) and 3.8 (0.9) at baseline, and 3.0 (2.3) and 2.0 (1.0) at 6-month follow-up, respectively. The optimal BASDAI cut-off values against external criteria were estimated to be < 1.4, < 2.8 and >5.9 (Table 1). Analyses on the level of agreement between BASDAI and ASDAS disease activity states demonstrated that the proportions of discordance decreased from 32.6% to 27.8% in baseline data and from 36.4% to 32.9% in follow-up data, when the estimated BASDAI cut-offs (< 1.4, < 2.8 and >5.9) were applied instead of the unvalidated BASDAI cut-offs (< 2, < 4 and >6) (Figure 1). The corresponding weighted kappa values increased slightly, but only a fair level of agreement was observed. The level of agreement between BASDAI and ASDAS disease activity states was superior at baseline compared to follow-up.
Conclusion: We estimated BASDAI cut-offs for the disease activity states against predefined external criteria to be < 1.4, < 2.8 and >5.9 in a large European observational cohort of patients with axSpA. Moderate agreement between disease activity states according to BASDAI and ASDAS was observed, which reflects that the underlying domains of BASDAI and ASDAS are not identical.
References:
1. Machado et al. (2011). Ann Rheum Dis, 70(1), 47-53.
To cite this abstract in AMA style:
Georgiadis S, Oernbjerg L, Michelsen B, Kvien T, Rasmussen S, Závada J, Bubová K, Glintborg B, Loft A, Rodrigues A, Santos M, Ciurea A, Nissen M, Kuusalo L, Rutanen J, Rotar Z, Perdan-Pikmajer K, Gudbjornsson B, Palsson O, DiGuiseppe D, Ostergaard M, Hetland M. Defining BASDAI Cut-offs for Disease Activity States in Axial Spondylarthritis – Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/defining-basdai-cut-offs-for-disease-activity-states-in-axial-spondylarthritis-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-basdai-cut-offs-for-disease-activity-states-in-axial-spondylarthritis-results-from-the-eurospa-collaboration/