Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous studies have suggested low-level chronic inflammation plays a key role in knee osteoarthritis (OA) development and progression, although attention has mostly been focused on localized articular tissues. In the present study, we performed deep immunophenotyping via mass cytometry to better characterize peripheral blood immunophenotype changes in OA patients, including progressor subetypes.
Methods: OA patient and control blood samples were obtained from the Oklahoma SOONER OA cohort and Oklahoma Immune Cohort, respectively. Radiographic OA was defined as baseline K/L grade 2-3 and knee pain on most days for the past 6 months. Fixed-flexion radiographs and WOMAC information from visits every 6 months was analyzed. Joint space width measurements were determined using Image Biospy Lab’s KOALA software. Radiographic progressors were defined as having mean medial TF joint space narrowing of ≥0.7mm within 24 months. Controls were matched for age ±5 years, ethnicity, BMI ±5 units, and sex. The final analysis included 21 OA patients (15 radiographic nonprogressors and 6 radiographic progressors) and 11 disease-free controls. Baseline blood samples were stained with Maxpar Direct immune profiling assay kit (Fluidigm), then analyzed using OMIQ. Cell cluster assignment and analysis analysis of 37 canonical immune cell populations was performed using OMIQ. Group differences were computed using the EdgeR algorithm (overdispersed Poisson model followed by empirical Bayes method) with P≤0.05 defining statistical significance.
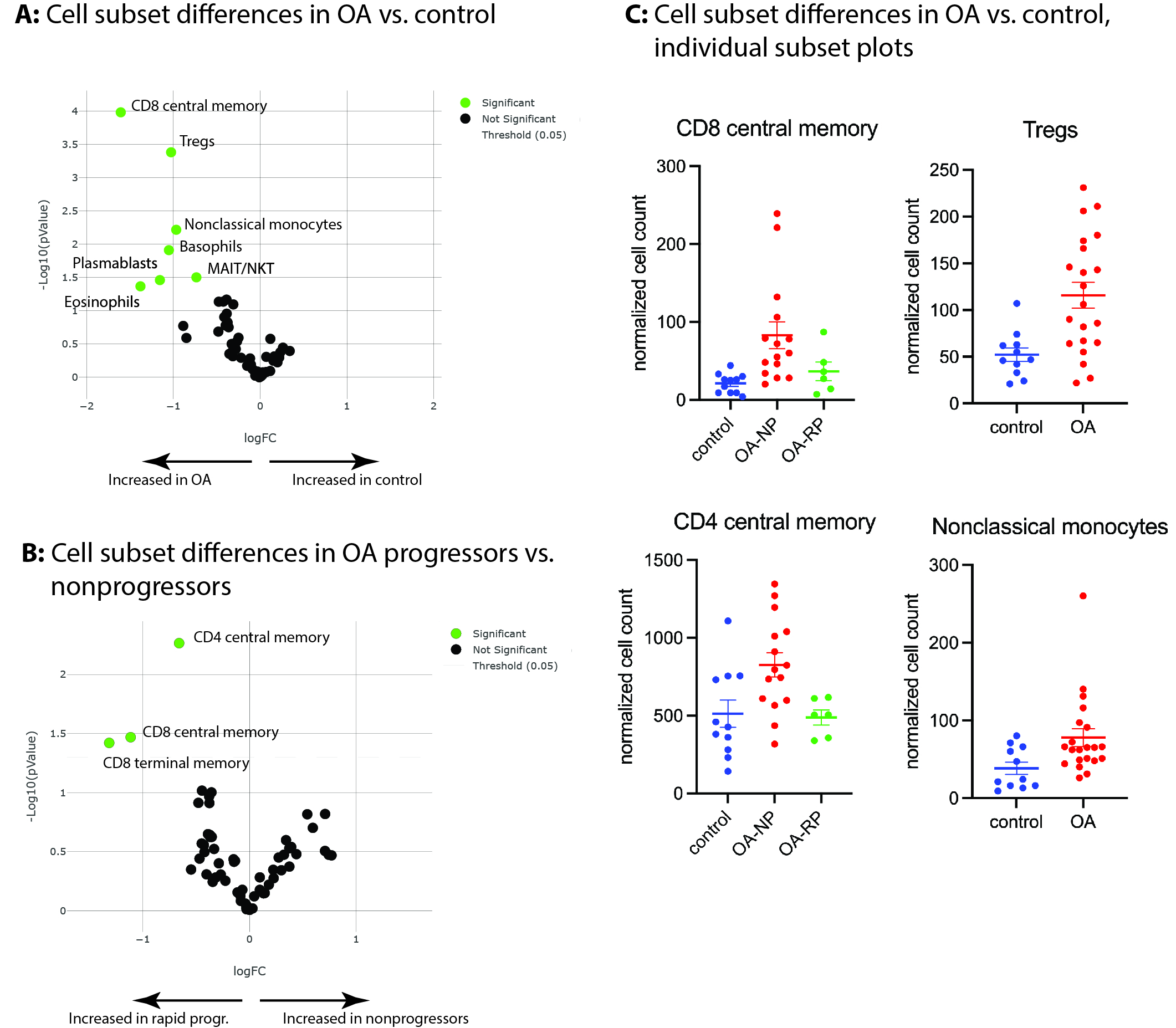
Results: OA patients were well matched with control subjects and among progressor groups, with no statistically significant differences in age, sex, BMI, baseline radiographic grade, or baseline WOMAC pain scores. Comparing all OA patients to controls using canonical cell subsets, we identified 7 subset differences (Figure 1A), with increases among OA patients in CD8 central memory cells (P=1E-4), Tregs (P=4E-4), and nonclassical monocytes (P=0.006), Figure 1A, 1C most prominent. Among OA patients comparing future radiographic progressors to future nonprogressors, we identified 3 subset differences, with decreases among rapid progressors in CD4 central memory cells (P=0.005), CD8 central memory cells (P=0.03), and CD8 terminal memory cells (P=0.03), Figure 1B.
Conclusion: Our study performed deep immunophenotyping of peripheral blood on a group of OA patients and well-matched controls. The results suggest that significant alterations in certain immune cell subpopulations are present among OA patients and at baseline prior to future radiographic progression and provide additional support for the hypothesis that OA represents a low-level systemic inflammatory disease. Future research should confirm our findings and investigate the pathophysiological consequences of these variations.
To cite this abstract in AMA style:
Barrett M, dyson G, Hannebutt N, Szymczak A, Miranda C, Jeffries M. Deep Immunophenotyping of Knee Osteoarthritis Patients Reveals Alterations in T Cell, Treg, and Monocyte Subpopulations [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/deep-immunophenotyping-of-knee-osteoarthritis-patients-reveals-alterations-in-t-cell-treg-and-monocyte-subpopulations/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deep-immunophenotyping-of-knee-osteoarthritis-patients-reveals-alterations-in-t-cell-treg-and-monocyte-subpopulations/