Session Information
Date: Saturday, November 6, 2021
Title: Spondyloarthritis Including PsA – Basic Science Poster (0046–0068)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Cross talk between different cell types, such as T cell subsets and other immune cell populations, is important in psoriatic disease. Imaging Mass Cytometry (IMC) is a novel technology that enables comprehensive analysis of cellular phenotypes and their interrelationships at the tissue level. We performed a multi-parameter characterization of various immune cell populations and assessed their spatial interactions in skin and synovial tissue samples from patients with psoriatic disease.
Methods: A total of 6 skin and 7 synovial formalin-fixed, paraffin embedded samples from patients with plaque psoriasis and psoriatic arthritis (PsA), respectively, were analyzed. Additionally, 1 synovial sample from a gout patients and a skin sample from a control patient were analyzed. We used a panel of 36 of metal-tagged antibodies to stain different cell populations. IMC was performed using the Hyperion Imaging System (Fluidigm). Tissue of 5-µm thickness was ablated with Nd:YAG 213 mm laser and analyzed in a Fluidigm CyTOF mass cytometer. Images were analyzed using MCD Viewer. Data were converted to TIFF format and segmented into single cells using previously developed methods. Individual cells were segmented using a combination of in-house program and CellProfiler to classify pixels on the basis of a combination of antibody stains to identify membranes and nuclei. The maps were then segmented into single-cell object masks using CellProfiler. Cell were classified into cell categories based on pre-specified markers (epithelial, vascular, stromal, myeloid and lymphoid) and further classified into immune cells types (T cell, macrophage, B cell, neutrophils, plasma cells). Single-cell marker expressions were summarized by mean pixel values for each channel. To visualize the number of cells per image the cell counts were normalized by the image area (total number of pixels) and displayed as cell density.
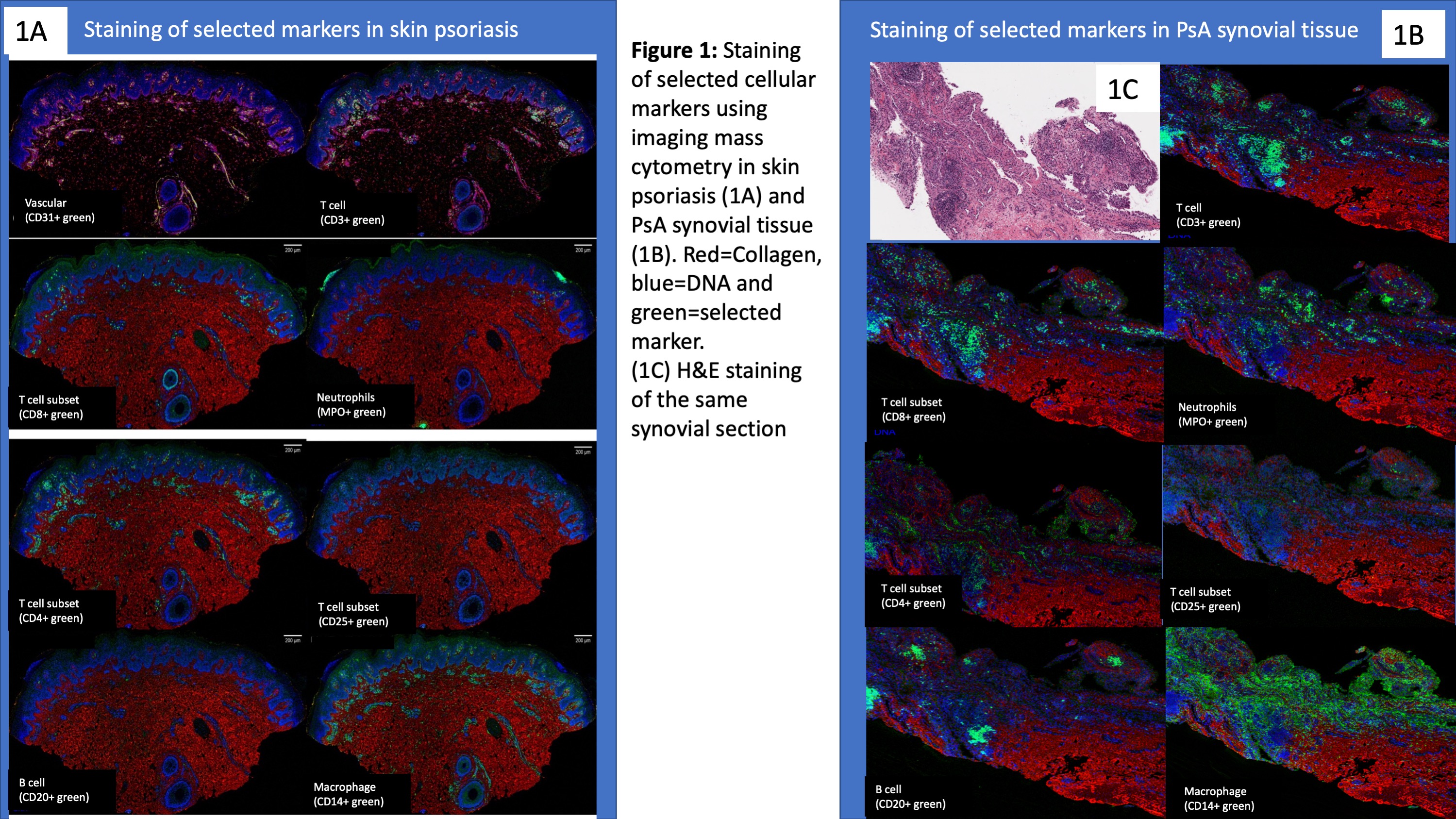
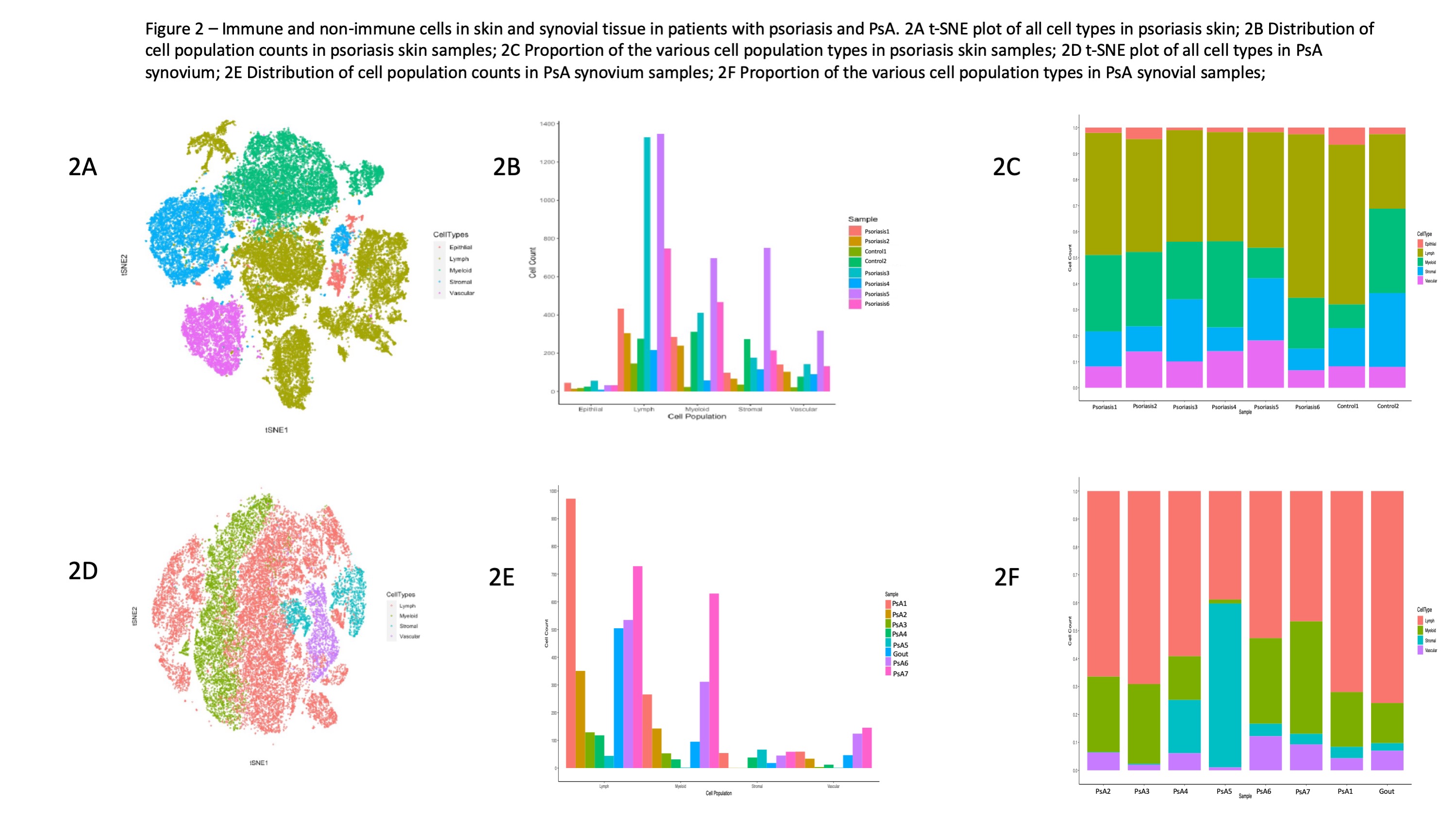
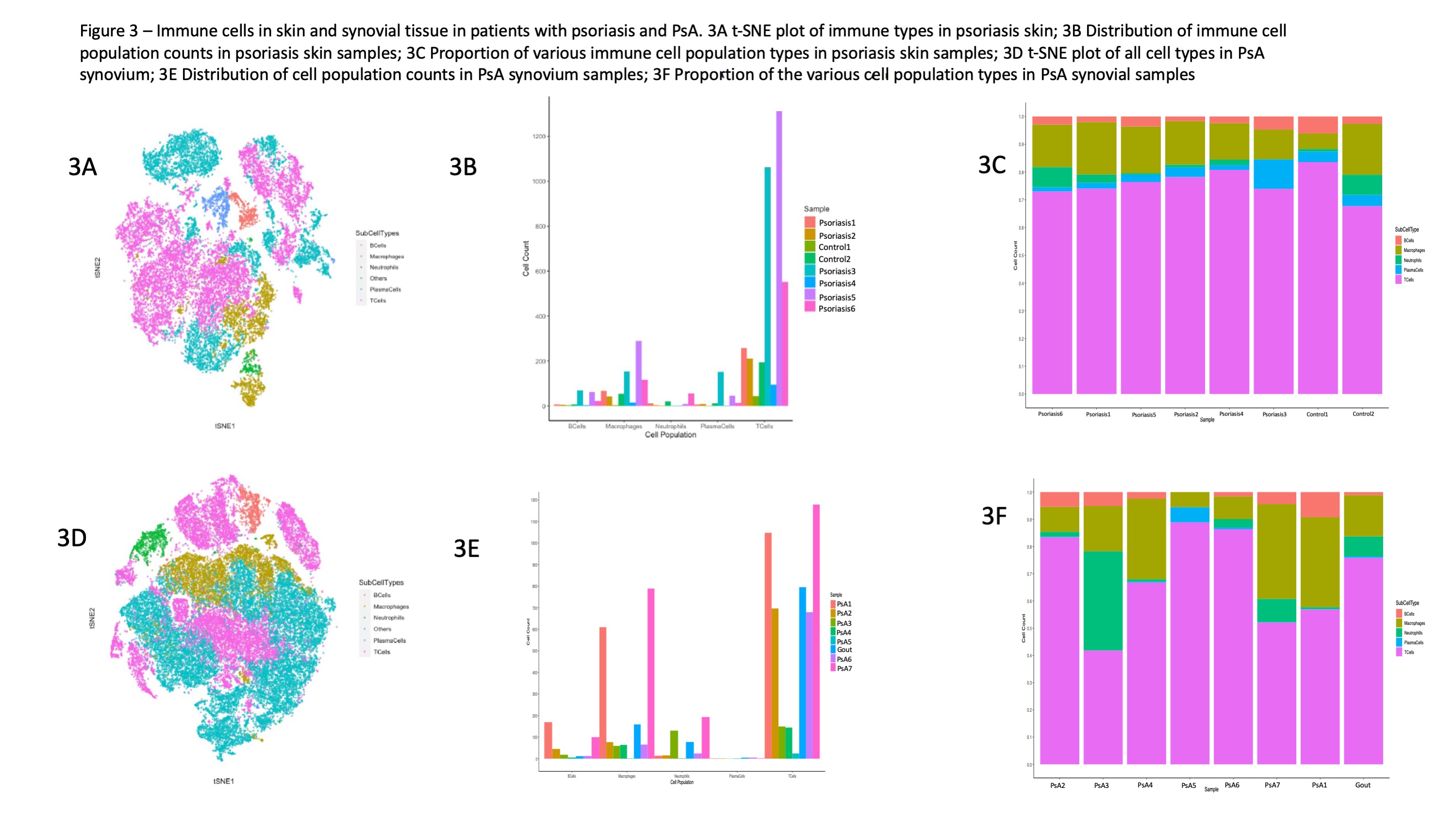
Results: Markers identifying stromal and immune cell types performed well and correlated with the expected location and morphological features of the various cell types (Figure 1). Among all cell types in the skin and synovium, lymphoid cells accounted for the most prevalent cell type followed by myeloid cells (Figure 2A-2B). Large degree of inter-patient heterogeneity was found in the prevalence of the various immune cell population across the different skin and synovial samples (Figure 3A-3B). T cells accounted for the largest immune cell type in both skin and synovial samples followed by macrophages and lower counts of neutrophils, B cells and plasma cells. Neighborhood analysis showed high correlation between CD20+, CD3+, CD4+ and CD8+ in the synovial tissue, which suggest close physical proximity and cross-talk between these T cell subsets and B cells in the synovium.
Conclusion: Innate and adaptive immune cells can be reliably identified using IMC in skin and synovial tissue. Cross talk between T cell subsets and innate immune cells in the skin and synovial tissue plays a role in psoriatic disease. IMC technology provides opportunities for exploring the underlying mechanisms driving psoriasis and PsA.
To cite this abstract in AMA style:
Eder L, Caucheteux S, Afiuni S, Krizova A, Limacher J, Jackson H, Piguet V. Deep Immune Cell Profiling of Tissue Microenvironment Using Imaging Mass Cytometry in Psoriatic Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/deep-immune-cell-profiling-of-tissue-microenvironment-using-imaging-mass-cytometry-in-psoriatic-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deep-immune-cell-profiling-of-tissue-microenvironment-using-imaging-mass-cytometry-in-psoriatic-disease/