Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Gastroesophageal reflux disease (GERD) is a common comorbidity in patients with SSc-ILD and may be associated with progression of SSc-ILD. In the SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) over 52 weeks by 44% versus placebo, with an adverse event profile characterized mainly by gastrointestinal events. We investigated the efficacy and safety of nintedanib in subgroups by presence of GERD at baseline.
Methods: In the SENSCIS trial, patients with SSc-ILD with first non-Raynaud symptom ≤7 years before screening, extent of fibrotic ILD ≥10% on HRCT and FVC ≥40% predicted were randomized to receive nintedanib or placebo until the last patient had reached week 52 but for ≤100 weeks. In post-hoc analyses, we analysed the annual rate of decline in FVC (mL/year) over 52 weeks and adverse events in subgroups by presence of GERD prior to and/or at screening. GERD was defined as present if it was noted as a present or past comorbidity on the SSc-related medical history page of the case report form.
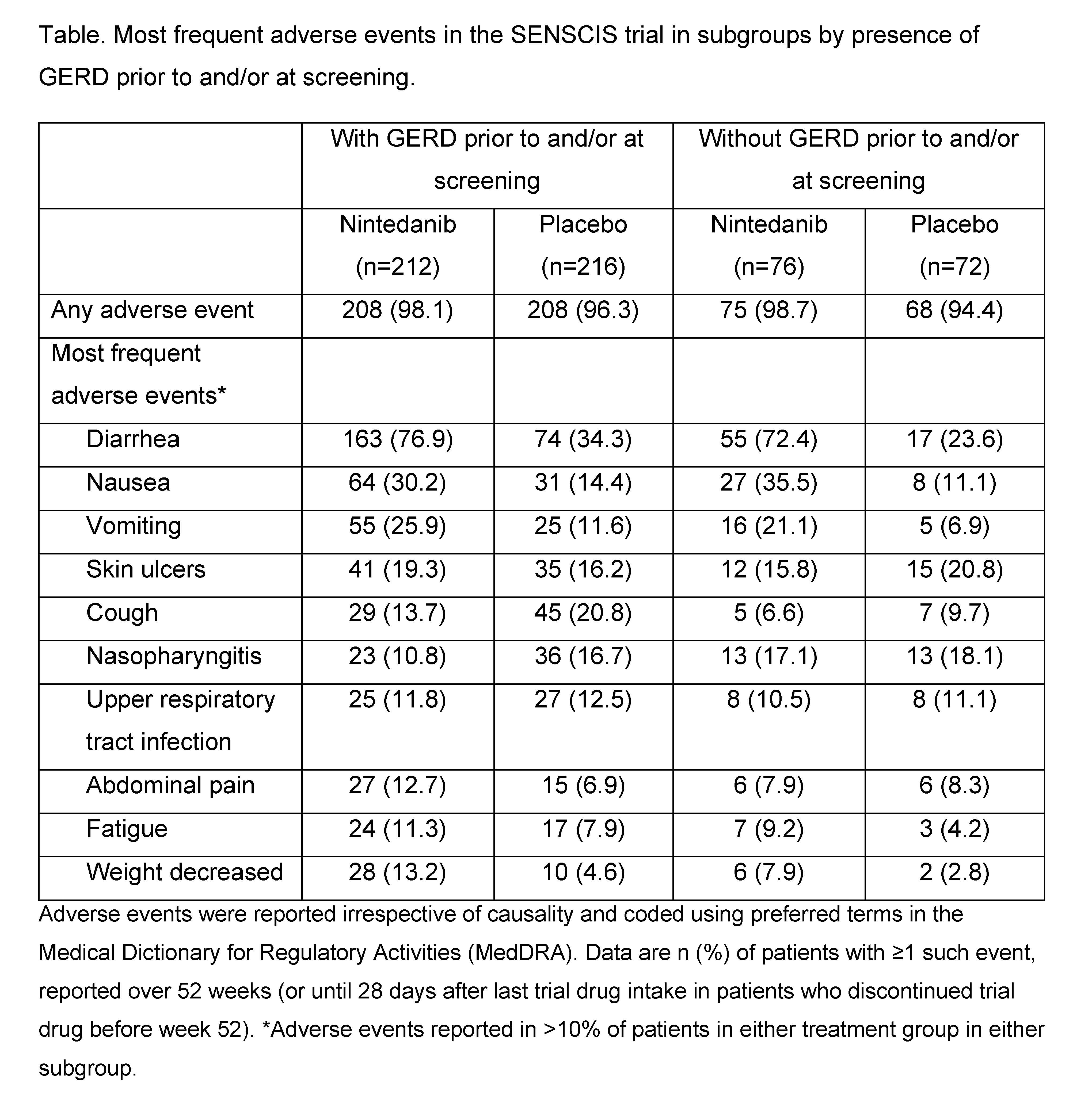
Results: Of 576 patients who received ≥1 dose of trial medication, 74.3% had GERD prior to and/or at screening. At baseline, in patients with and without GERD, respectively, mean (SD) FVC was 2532 (787) and 2405 (744) mL and 72.3 (16.7) and 73.1 (16.7) % predicted; 88.8% and 52.0% of patients were taking anti-acid therapy. In the placebo group, the adjusted annual rate of FVC decline was numerically more pronounced in patients with than without GERD (-97.3 vs -81.6 mL/year). The effect of nintedanib versus placebo on reducing the rate of FVC decline (mL/year) was numerically more pronounced in patients with than without GERD (difference: 48.2 [95% CI: 3.9, 92.5] vs 20.2 [-54.6, 95.0]), but the exploratory interaction p-value did not indicate heterogeneity in the treatment effect of nintedanib between these subgroups (p=0.53) (Figure). The most frequent adverse event reported in all the subgroups was diarrhea (Table). In the nintedanib and placebo groups, respectively, the proportions of patients who had adverse events leading to treatment discontinuation were 14.6% and 9.7% in patients with GERD and 19.7% and 5.6% in patients without GERD.
Conclusion: In the SENSCIS trial in patients with SSc-ILD, nintedanib slowed the rate of FVC decline versus placebo both in patients with and without GERD. The adverse event profile of nintedanib was similar in patients with and without GERD.
To cite this abstract in AMA style:
Highland K, Criner G, Sfikakis P, Nunes H, Stevens W, Miede C, Alves M, Kreuter M. Decline in Forced Vital Capacity in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) with and Without Gastroesophageal Reflux Disease: Further Analyses of the SENSCIS Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/decline-in-forced-vital-capacity-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-with-and-without-gastroesophageal-reflux-disease-further-analyses-of-the-senscis-tr/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/decline-in-forced-vital-capacity-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-with-and-without-gastroesophageal-reflux-disease-further-analyses-of-the-senscis-tr/