Session Information
Session Type: Late-Breaking Abstracts
Session Time: 8:00AM-9:30AM
Background/Purpose: Dapirolizumab pegol (DZP) is a novel, polyethylene glycol (PEG)-conjugated antigen-binding (Fab’) fragment, lacking an Fc domain, that inhibits CD40L signaling. By binding to CD40L, DZP has broad modulatory effects on systemic lupus erythematosus (SLE) immunopathology, including reducing B and T cell activation and downregulating interferon pathways.1,2 The phase 3 PHOENYCS GO trial (NCT04294667) evaluated the efficacy and safety of DZP in patients (pts) with moderate-to-severe SLE.
Methods: PHOENYCS GO was a 48-week (wk), randomized, placebo (PBO)-controlled trial. After the treatment period pts could enter an open-label extension or complete a 6-wk safety follow-up. Pts aged ≥16 years with moderate-to-severe SLE characterized by persistently active or frequently flaring/relapsing-remitting disease activity despite stable standard of care (SOC) medication (antimalarials, corticosteroids, and/or immunosuppressants) were included. Pts were randomized 2:1 to intravenous DZP 24 mg/kg plus SOC medication (DZP+SOC) or PBO+SOC every 4 wks. The primary endpoint was British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) response at Wk 48. Secondary endpoints included SLE Responder Index (SRI)-4 response at Wk 48 and prevention of severe BILAG flares through Wk 48.
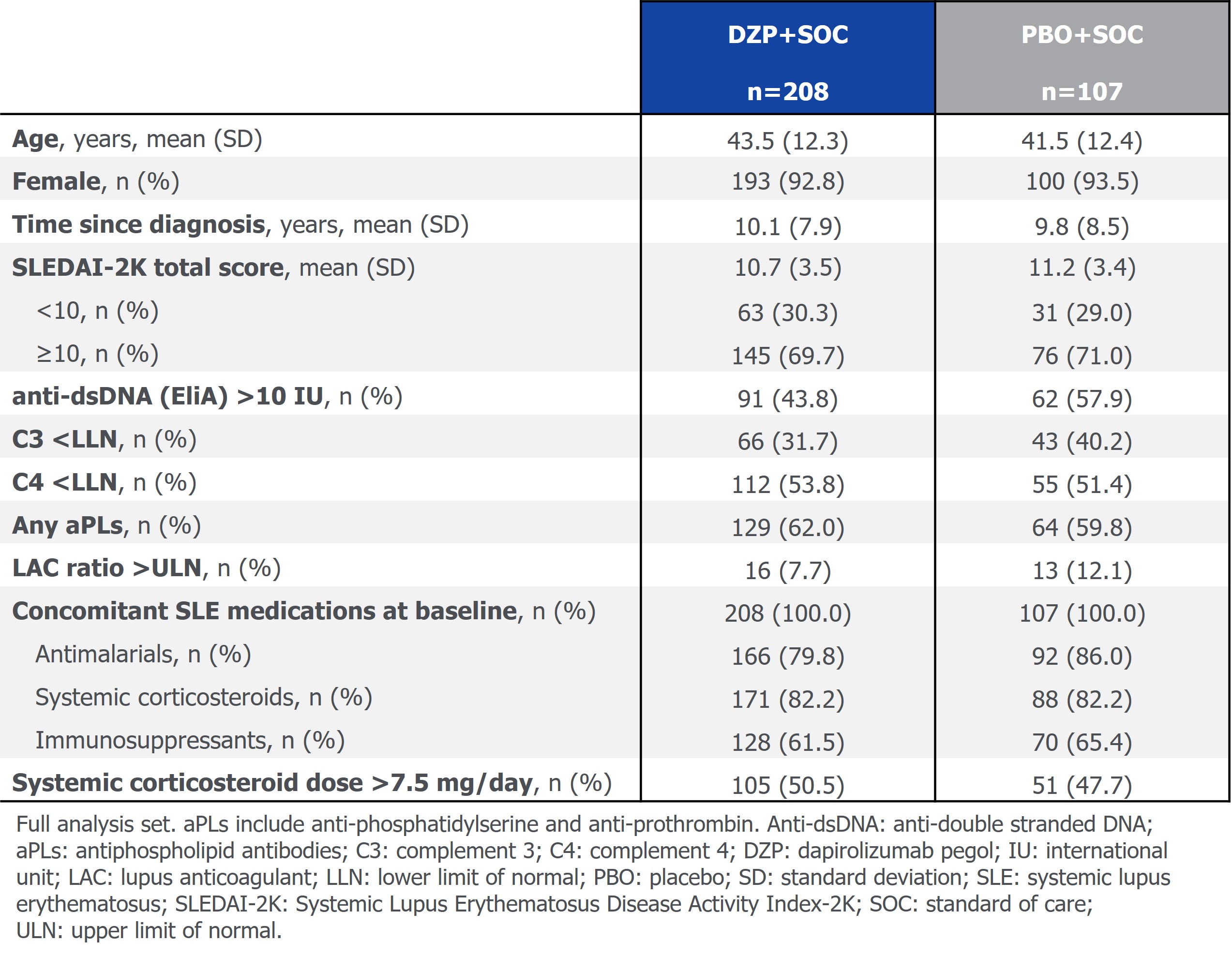
Results: Overall, 90.1% of pts receiving DZP+SOC and 84.3% receiving PBO+SOC completed the study to Wk 48. Baseline characteristics were generally similar between treatment groups (Table).
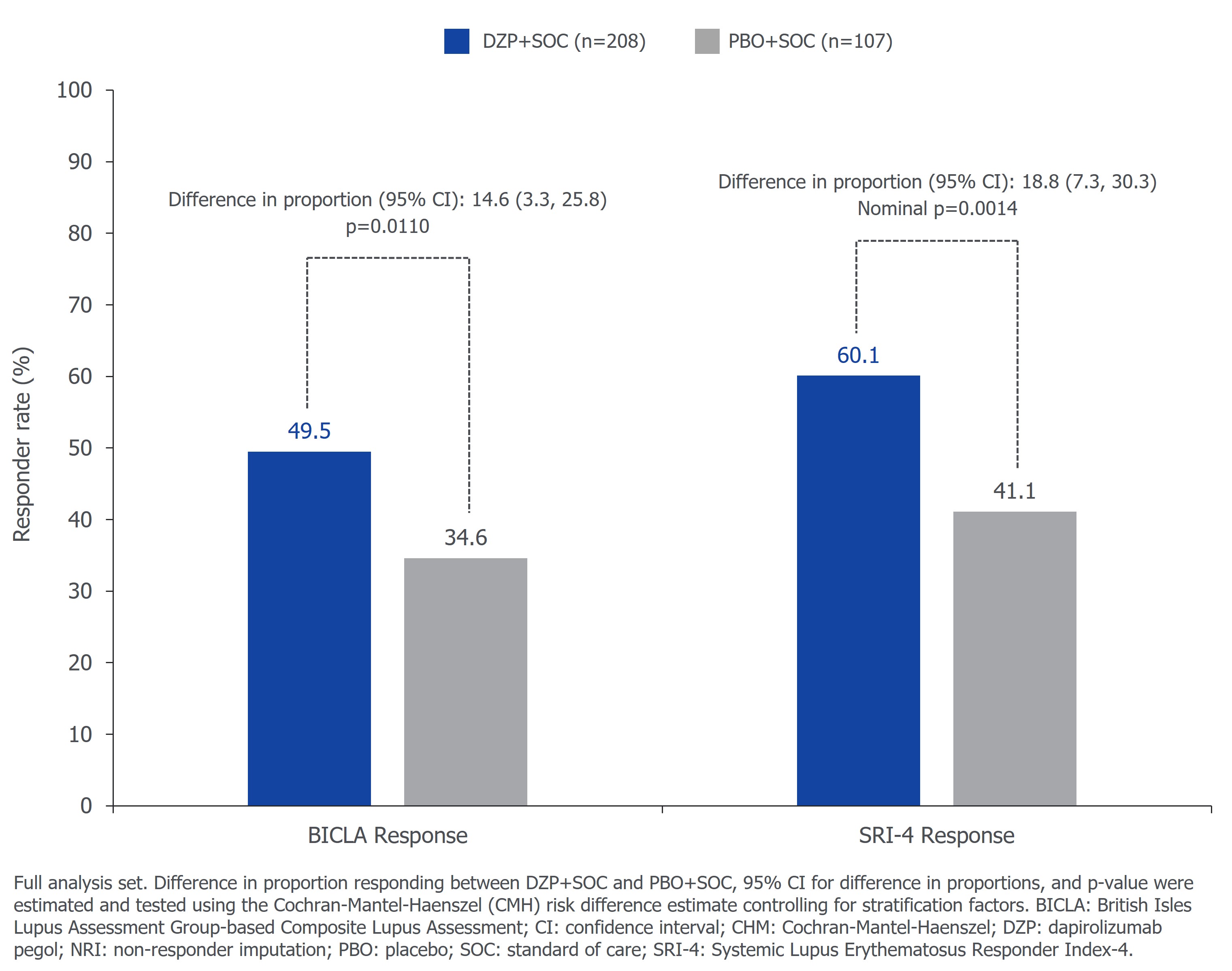
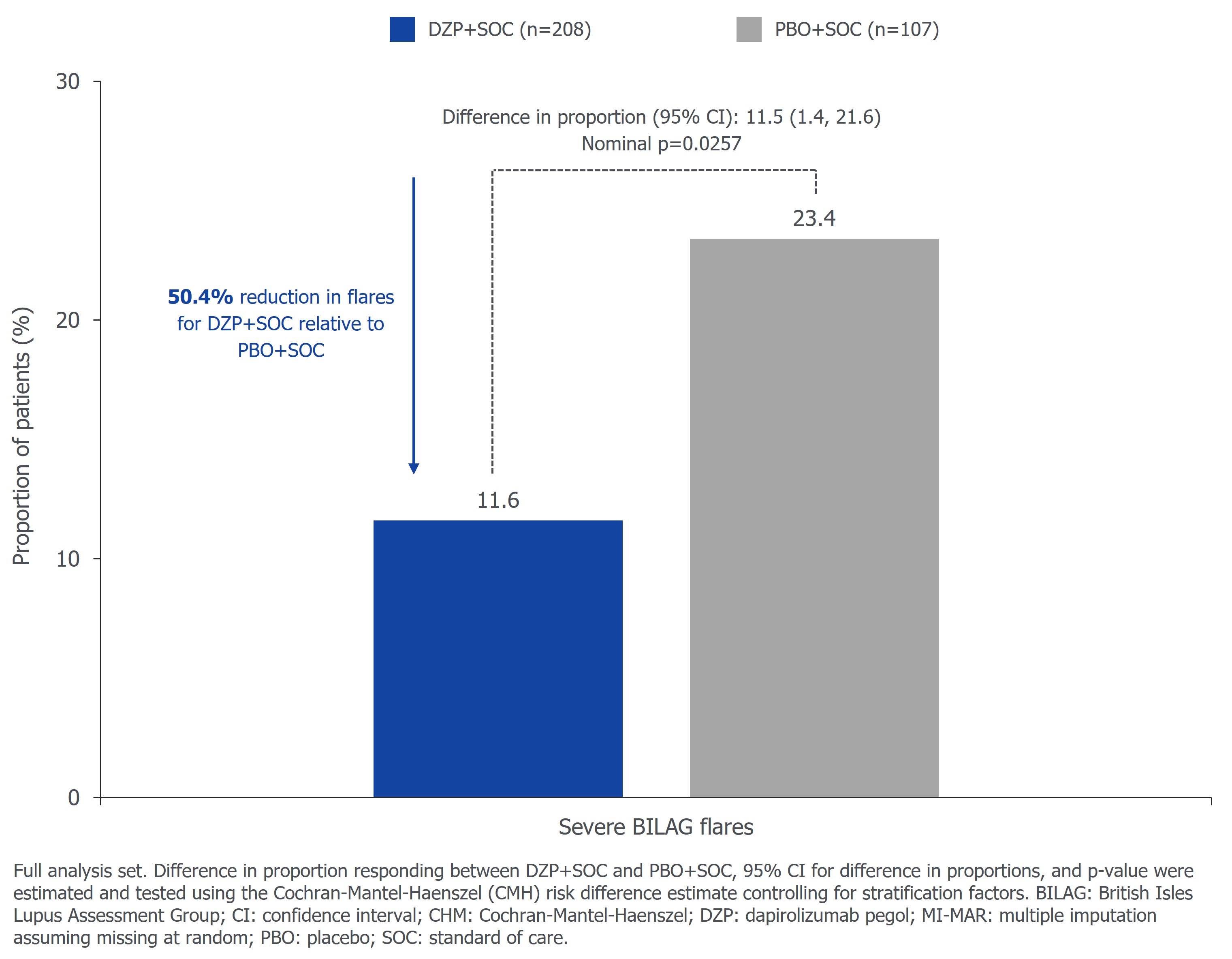
The primary endpoint was met; 49.5% (103/208) vs 34.6% (37/107) of pts receiving DZP+SOC vs PBO+SOC had BICLA response at Wk 48 (p=0.0110; difference 14.6%; Figure 1). With respect to SRI-4, 60.1% (125/208) vs 41.1% (44/107) of pts receiving DZP+SOC vs PBO+SOC had response at Wk 48 (nominal p=0.0014; difference 18.8%; Figure 1). 11.6% vs 23.4% of pts receiving DZP+SOC vs PBO+SOC had severe BILAG flares through Wk 48 (nominal p=0.0257; difference 11.5%; Figure 2). Per protocol, pts with a corticosteroid dose >7.5 mg/day prednisone equivalent were required to start tapering no later than Wk 8 to reach ≤7.5 mg/day. In pts with corticosteroid dose >7.5 mg/day at baseline, 72.4% (76/105) vs 52.9% (27/51) of pts receiving DZP+SOC vs PBO+SOC reduced their dose to ≤7.5 mg/day at Wk 48 (nominal p=0.0404; difference 17.1%).
A higher proportion of pts receiving DZP had ≥1 treatment-emergent adverse event (TEAE; DZP+SOC: 82.6%; PBO+SOC: 75.0%); however, the proportion of pts with serious TEAEs was lower (DZP+SOC: 9.9%; PBO+SOC: 14.8%). Opportunistic infections were reported in 2.8% and 0.9% of pts receiving DZP+SOC and PBO+SOC, respectively. There was 1 thromboembolic TEAE (myocardial infarction) and 1 death (due to gangrene-related sepsis) in pts with predisposing medical history receiving DZP+SOC.
Conclusion: Treatment with DZP, a novel CD40L inhibitor, resulted in improvement in disease activity and corticosteroid tapering in pts with SLE; significantly more pts who received DZP+SOC achieved BICLA response vs PBO+SOC. DZP was generally well tolerated.
References: 1. Cutcutache I. Arthritis Rheumatol 2023;75 (suppl 9). 2. Powlesland A. Annals Rheum Dis 2024;83 (suppl 1):261.
To cite this abstract in AMA style:
Clowse M, Isenberg D, Merrill J, Dörner T, Petri M, Vital E, Morand E, Jimenez T, Brookes S, Gaiha-Rohrbach J, Martin C, Nelde A, Stach C. Dapirolizumab Pegol Demonstrated Significant Improvement in Systemic Lupus Erythematosus Disease Activity: Efficacy and Safety Results of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/dapirolizumab-pegol-demonstrated-significant-improvement-in-systemic-lupus-erythematosus-disease-activity-efficacy-and-safety-results-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dapirolizumab-pegol-demonstrated-significant-improvement-in-systemic-lupus-erythematosus-disease-activity-efficacy-and-safety-results-of-a-phase-3-trial/