Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Detection of urinary CXCL6, a member of the IL-8 chemokine family, has been linked to CKD and is a proposed marker of chronic damage in IgA nephropathy. While a direct role for lung epithelial derived CXCL6 on fibroblast activation has been described in pulmonary fibrosis, the mechanism by which CXCL6 may promote fibrotic changes in the kidney remains poorly characterized. Accordingly, this study leveraged the Accelerating Medicines Partnership (AMP) and NYU lupus nephritis (LN) cohorts to evaluate whether renal fibrosis associates with CXCL6 production by kidney epithelium, and if CXCL6 can activate renal fibroblasts in vitro.
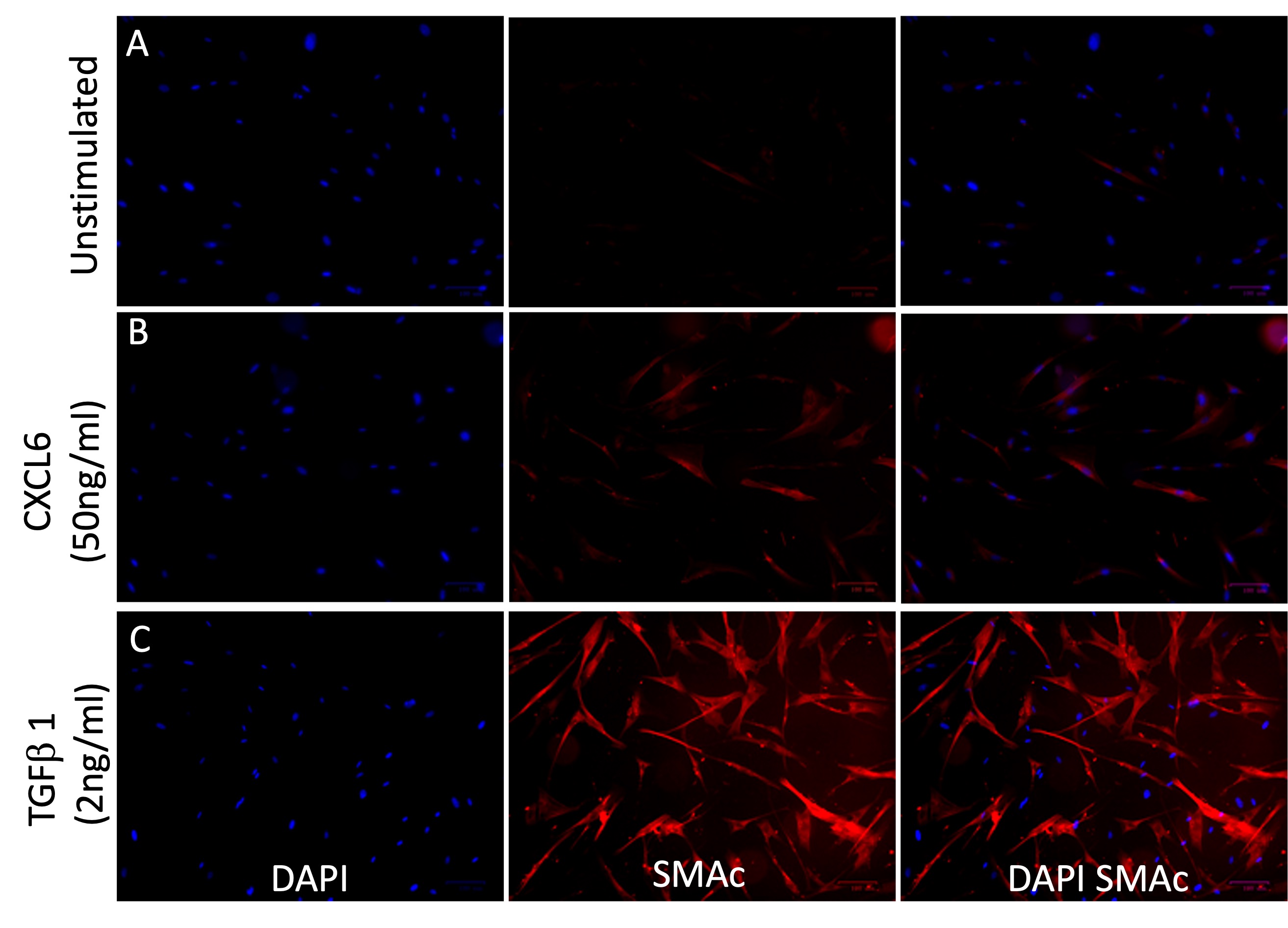
Methods: LN patients were consecutively enrolled in AMP at the time of a renal biopsy and followed for one year. Dissociated biopsies were passed through a single-cell RNA sequencing (scRNAseq) pipeline and 1200 proteins were quantified in urine collected within 3 weeks of biopsy and at each visit thereafter. Additional biospecimens (urine, plasma, and kidney tissue) were prospectively collected from SLE patients in the NYU lupus cohort to corroborate AMP findings. Primary renal fibroblasts were stimulated in vitro with recombinant CXCL6 for 72 hours and SMAc induction was visualized with immunofluorescence.
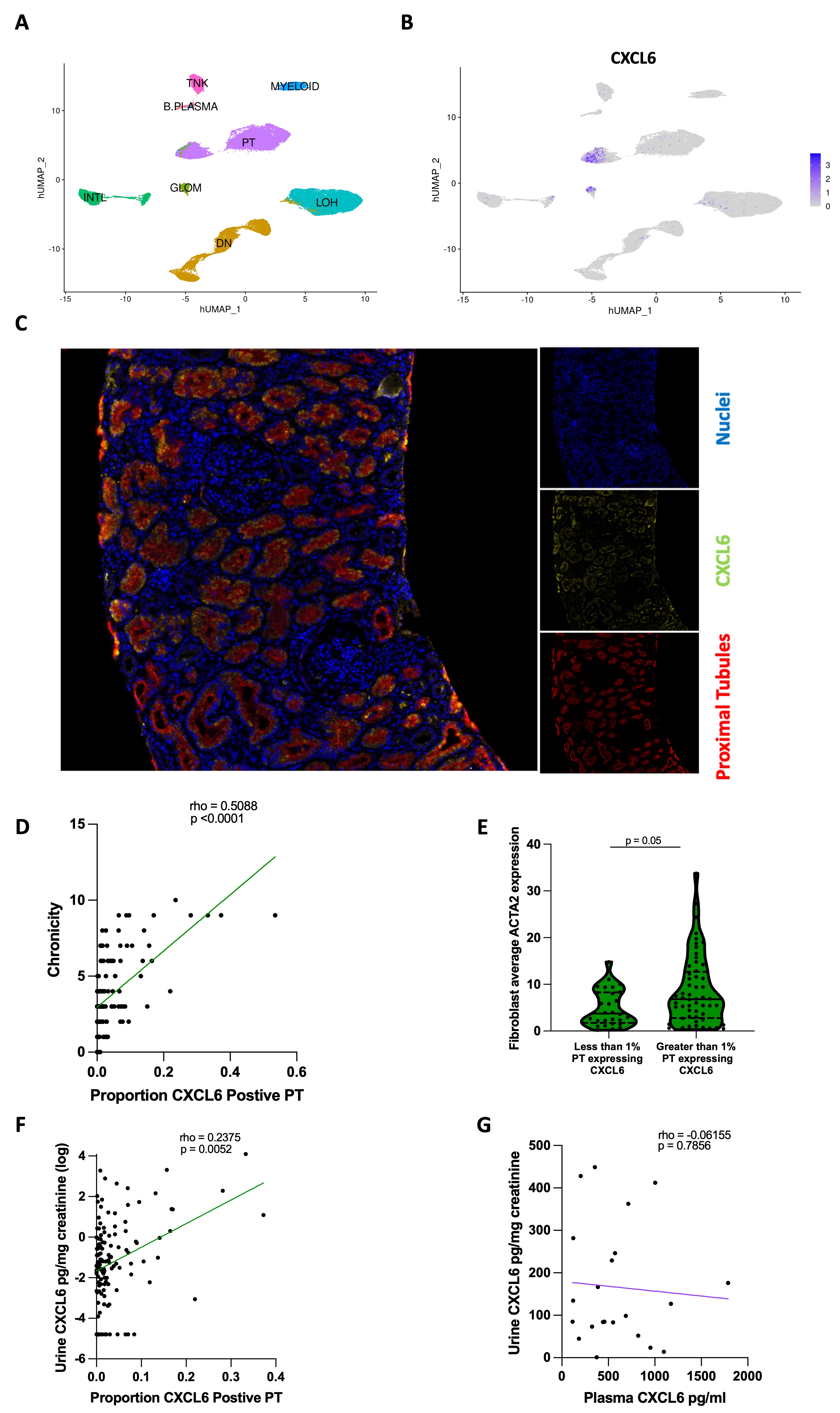
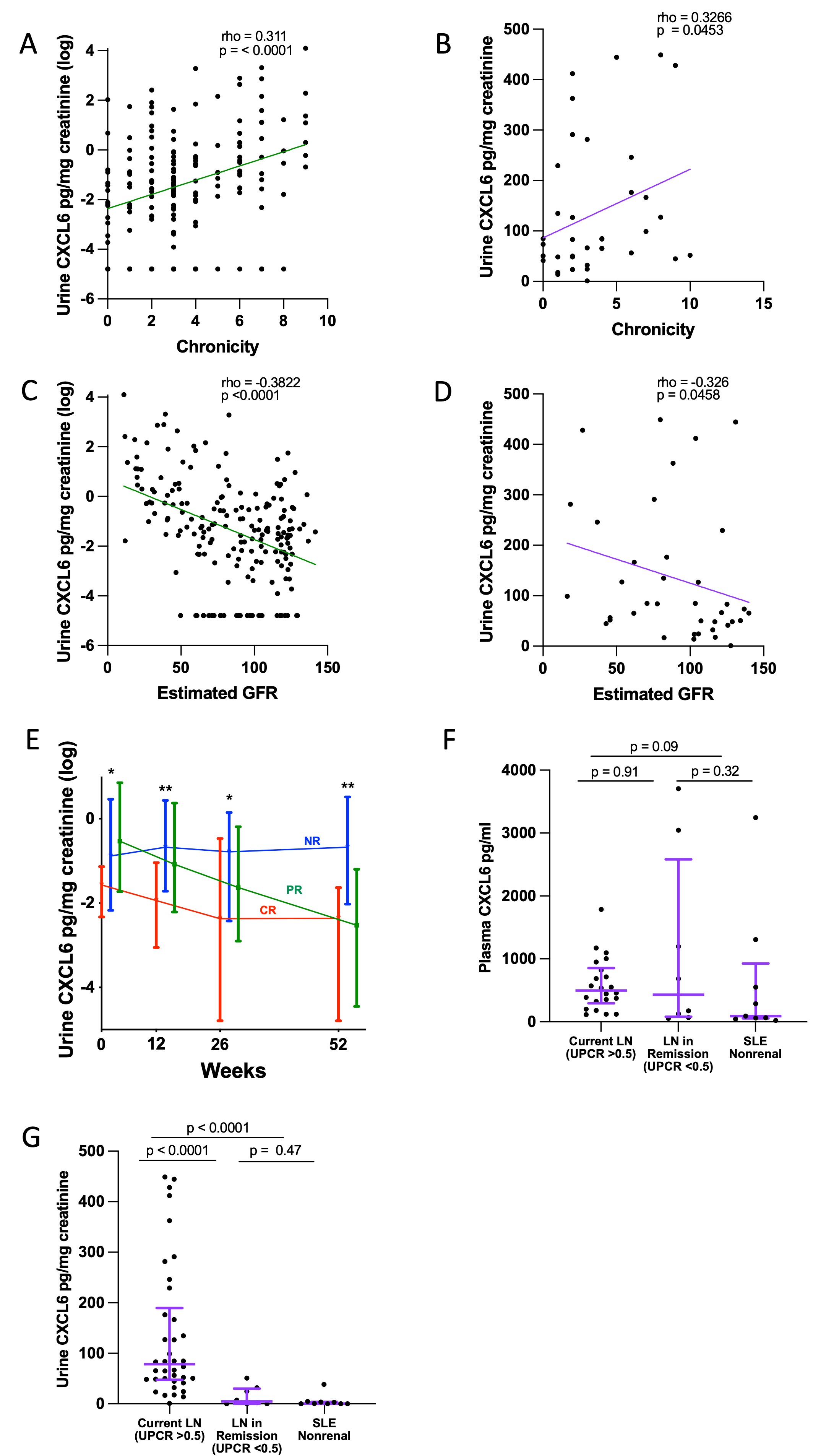
Results: The AMP scRNAseq revealed expression of CXCL6 by proximal tubule (PT) cells and PT CXCL6 protein expression was confirmed by immunofluorescence (Fig 1A-C). The proportion of PT cells expressing CXCL6 significantly associated with biopsy chronicity (Fig 1D). Fibroblasts from biopsies with greater than 1% of PT cells expressing CXCL6 had higher average ACTA2 expression suggesting myofibroblast transdifferentiation (Fig 1E). The proportion of CXCL6 expressing PT cells significantly associated with matched urinary CXCL6 levels, but there was no correlation between CXCL6 quantified in matched plasma and urine samples from LN collected at NYU, suggesting intrarenal production as the source of urinary CXCL6 (Fig 1F-G). Urinary CXCL6 correlated with higher chronicity and decreased eGFR in both the AMP and NYU cohorts (Fig 2A-D). In the AMP study, patients classified as nonresponders to standard of care at 52 weeks had higher urinary CXCL6 at baseline, 12, 26, and 52 weeks compared to complete responders (Fig 2E). In the NYU cohort, urinary but not plasma CXCL6 was significantly higher in patients with current LN (UPCR >0.5) compared to LN patients in remission (UPCR < 0.25/normal creatinine, median time from biopsy = 9 years) and SLE nonrenal patients (Fig 2F-G). The association with current LN vs LN in remission/SLE nonrenal (as a combined variable) persisted after adjusting for GFR in a logistic regression (p=0.001). Stimulation of primary renal fibroblasts in vitro with recombinant CXCL6 resulted in the expression of SMAc protein suggesting fibroblast activation.
Conclusion: CXCL6 secretion by PT cells associates with LN biopsy chronicity and is higher in SLE patients with persistent proteinuria. Further, CXCL6 treatment results in renal myofibroblast transdifferentiation in vitro. These data suggest that PT to fibroblast crosstalk through a CXCL6 axis contributes to renal fibroblast activation and irreversible scar formation in LN.
To cite this abstract in AMA style:
Carlucci P, Sachan N, Fava A, Cohen B, Shwetar J, Gurajala S, Xiao Q, Mears J, Preisinger K, Zaminski D, Deonaraine K, Izmirly P, James J, Guthridge J, DeJager W, Wofsy D, Loomis C, Ward G, Wu M, Putterman C, Rao D, Diamond B, Fine D, Monroy-Trujillo J, Belmont H, Apruzzese W, Davidson A, Furie R, Hoover P, Berthier C, Dall'Era M, Kamen D, Kalunian K, Anolik J, Barnas J, Arazi A, Raychaudhuri S, Hacohen N, Clancy R, Ruggles K, Petri M, Buyon J. CXCL6 Synthesized by Proximal Tubule Cells May Promote Fibrosis in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cxcl6-synthesized-by-proximal-tubule-cells-may-promote-fibrosis-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cxcl6-synthesized-by-proximal-tubule-cells-may-promote-fibrosis-in-lupus-nephritis/