Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Safe and effective treatments are of significant unmet need in DM. Lenabasum, a CB2 agonist that activates resolution of inflammation, improved skin disease, patient-reported outcomes, and biomarkers in a Phase 2 study of DM patients with active skin disease.
Methods: DM patients ≥ 18 years old with active skin with or without muscle involvement were enrolled in 55 sites in North America, Europe, and Asia-Pacific. Stable doses of background immunosuppressant were allowed. Subjects were randomized 2:1:2 to lenabasum 20 mg BID, lenabasum 5 mg BID, or placebo BID for 52 weeks, with visits ≤ 8 weeks apart. The study was stopped after all subjects completed Week 28. Some subjects had completed Week 52 by then. The primary efficacy endpoint was Total Improvement Score (TIS) at Week 28 and a secondary efficacy endpoint was TIS at Week 52, for lenabasum 20 mg BID vs placebo.
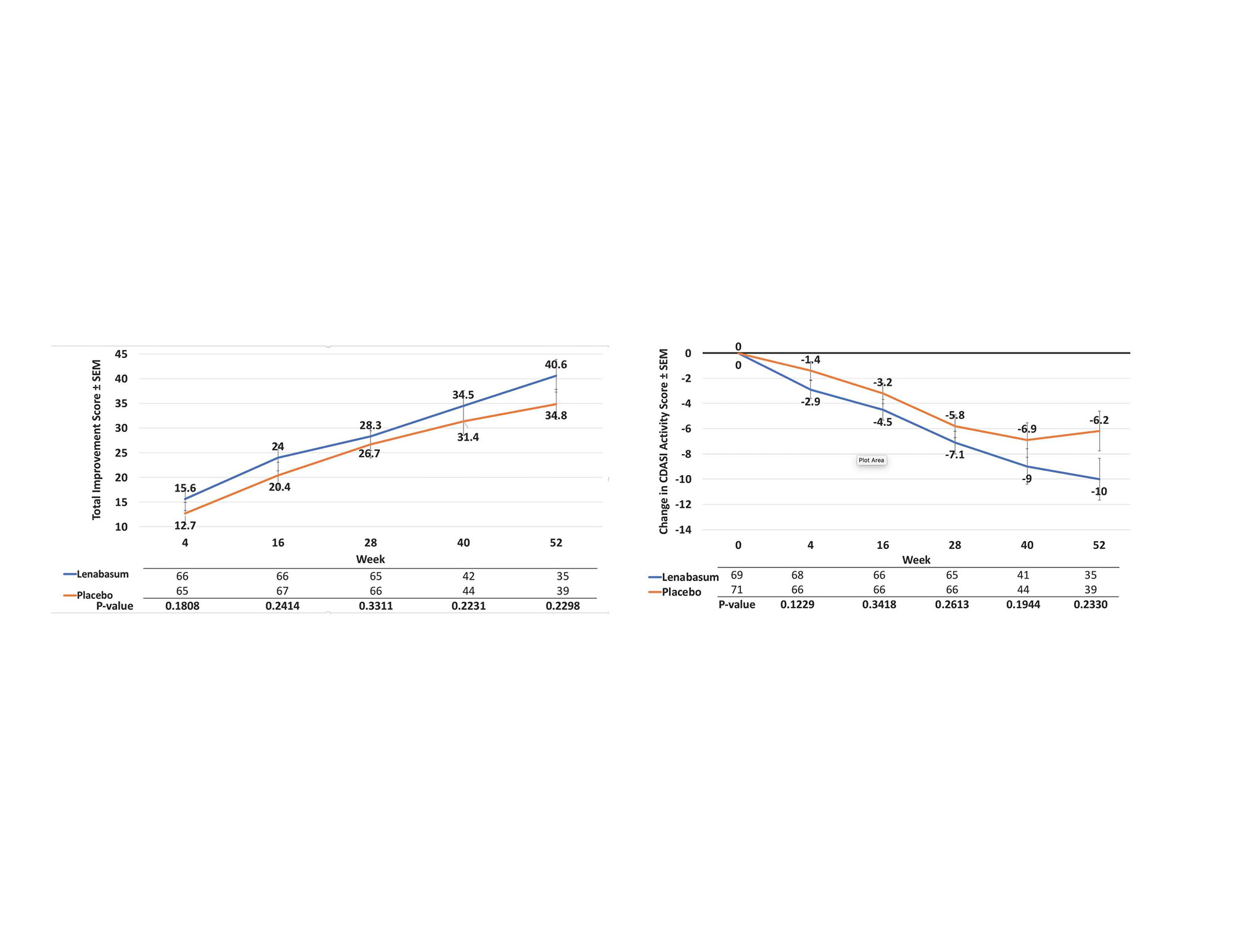
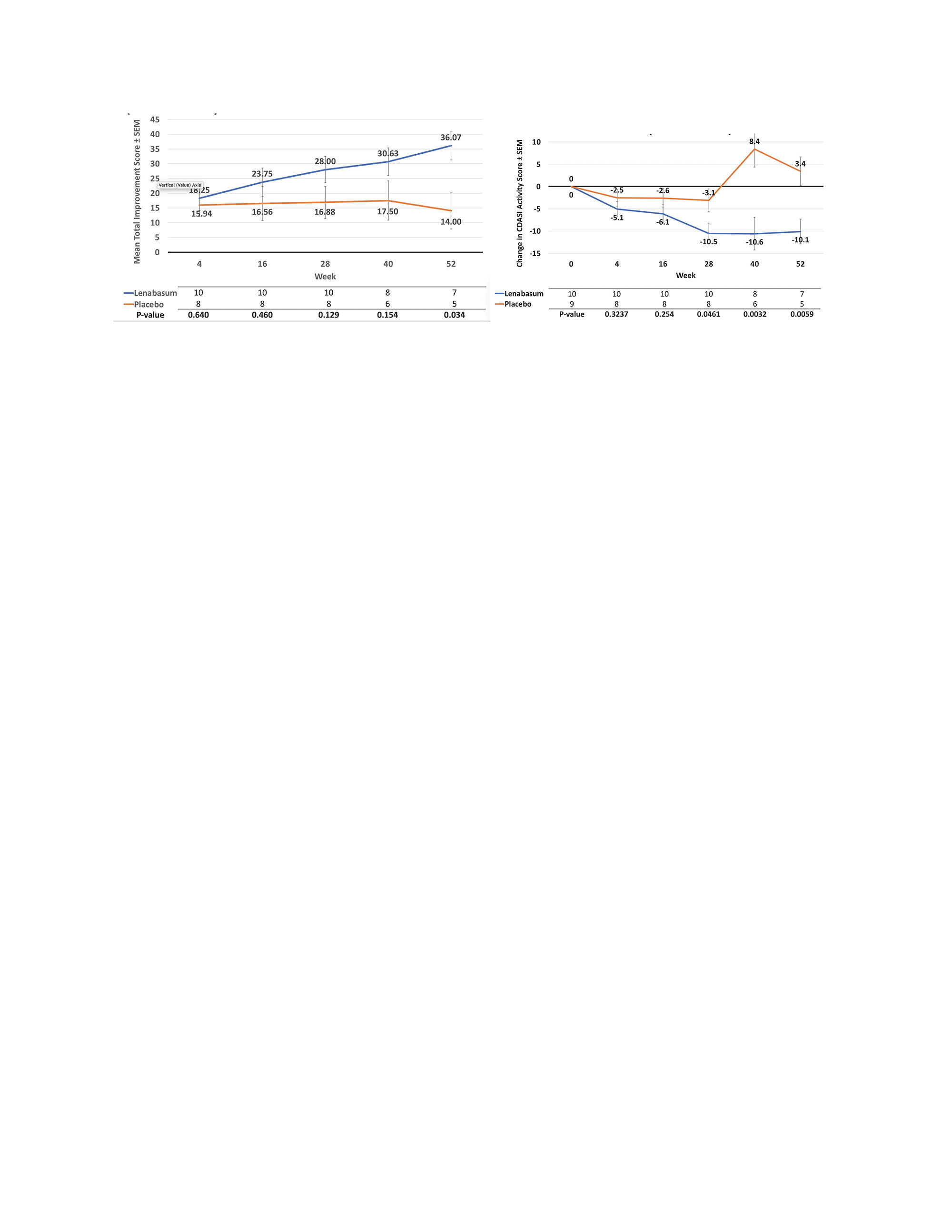
Results: 175 subjects (69 lenabasum 20 mg BID, 35 lenabasum 5 mg BID, 71 placebo BID) received study drug; 167 completed Week 28, and 103 completed Week 52. The most common reasons for study discontinuation were study stopped by Sponsor (34.3%), withdrawal of consent (4.5%), and adverse events (AEs, 3.9%), with similar rates among groups. Baseline demographics and disease measurements were similar among groups. The primary efficacy endpoint was not met – mean (SD) TIS score was 28.3 (19.75) vs 27.2 (19.23) at Week 28 for lenabasum 20 mg BID vs placebo, p = 0.3311, MMRM. Week 52 values were 40.6 (16.88) vs 34.8 (19.94), p = 0.2290. When analyses were restricted to subjects with muscle weakness at baseline (MMT8 < 142), TIS scores and treatment differences were greater and reached nominal statistical significance at Week 40, p = 0.0172. Mean (SD) improvements in CDASI activity score were numerically greater but not statistically different between lenabasum 20 mg BID group vs placebo at Week 28 [-7.1 (7.76) vs -5.8 (8.88) points, p = 0.2775] and Week 52 [-10.0 (9.45) vs -6.2 (12.8) points, p = 0.0932]. When restricting analysis of participants without muscle weakness (MMT-8 = 150), improvement in CDASI activity score was greater in the lenabasum 20 mg BID group vs placebo at Week 28, p = 0.0461, and Week 52, p = 0.0059. Treatment-emergent AEs (TEAEs) occurred in 87.0%, 85.7%, and 87.3% of lenabasum 20 mg BID, lenabasum 5 mg BID, and placebo groups, with no deaths. Related TEAEs leading to withdrawal of study product were infrequent. Serious TEAEs occurred in 11.6%, 8.6%, and 4.2% of subjects in the lenabasum 20 mg BID, lenabasum 5 mg BID, and placebo groups. No serious TEAE preferred term occurred in more than 1 subject in any group. TEAE occurring in ≥ 10% of lenabasum 20 mg BID subjects were (% lenabasum vs % placebo): dermatomyositis (flare) 27.5% vs 40.8%; diarrhea 14.5% vs 8.5%; dizziness 13.0% vs 4.2%; nausea 11.6% vs 4.2%; headache 10.1% vs 14.1%; and arthralgia 10.1% vs 2.8%.
Conclusion: Although, primary or secondary endpoints were not met in the study, subgroup analysis of patients with muscle weakness and without muscle weakness, showed improvement in muscle strength and rash, respectively in lenabasum 20 mg BID group vs placebo. Lenabasum was administered safely and was well-tolerated in this study.
To cite this abstract in AMA style:
Werth V, White B, Concha J, Dan J, Dgetluck N, Hally K, Constantine S, Aggarwal R, Fiorentino D, Lundberg I, Oddis C. Cutaneous Manifestations, Clinical Trials, Safety Efficacy and Safety of Lenabasum in the Phase 3 DeterMine Trial in Dermatomyositis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cutaneous-manifestations-clinical-trials-safety-efficacy-and-safety-of-lenabasum-in-the-phase-3-determine-trial-in-dermatomyositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cutaneous-manifestations-clinical-trials-safety-efficacy-and-safety-of-lenabasum-in-the-phase-3-determine-trial-in-dermatomyositis/