Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Modified Rodnan Skin Score is a widely used outcome measure for skin involvement in systemic sclerosis (SSc). Its criterion validity (comparison to the gold standard) was originally established by Dr. Rodnan by comparing the clinical skin scoring with the weight of punch skin biopsy performed on the dorsal surface of the forearm in patients with diffuse cutaneous involvement. A comparison to the histological evaluation of punch skin biopsies was not performed. Moreover, the words “skin thickening” and “skin induration” (hardening) are used interchangeably when referring to the construct captured by mRSS. However, dermal thickness and hardening (also influenced by extracellular matrix density) are non-overlapping constructs. Lastly, mRSS is used to evaluate skin involvement in limited and diffuse cutaneous subsets in routine clinical care, although the criterion validity of mRSS in the limited cutaneous subset has not yet been established. Herein, we investigated the correlation of clinical local skin scoring and total mRSS with histological measures in forearm skin biopsies of SSc patients.
Methods: 55 SSc patients were included, consisting of 39 with a diffuse subtype (including 13 with early disease [disease duration < 3 years]) and 16 with limited cutaneous involvement (including 6 with early disease). All clinical skin scorings were performed by one experienced SSc expert. A 3-mm punch skin biopsy was taken from the dorsal surface of the forearm. Special attention was given to performing a full-thickness skin biopsy with the inclusion of the fat layer. Histological evaluation was performed in a blinded fashion. It included measurement of dermal thickness on H&E staining (from dermo-epidermal junction to the beginning of subcutaneous fat layer), automated assessment of collagen density in the dermis on Masson trichrome staining using Image J, and a semi-quantitative assessment of skin fibrosis by an SSc skin pathology expert (ranging from 0 [normal] to 3 [severely fibrotic]).
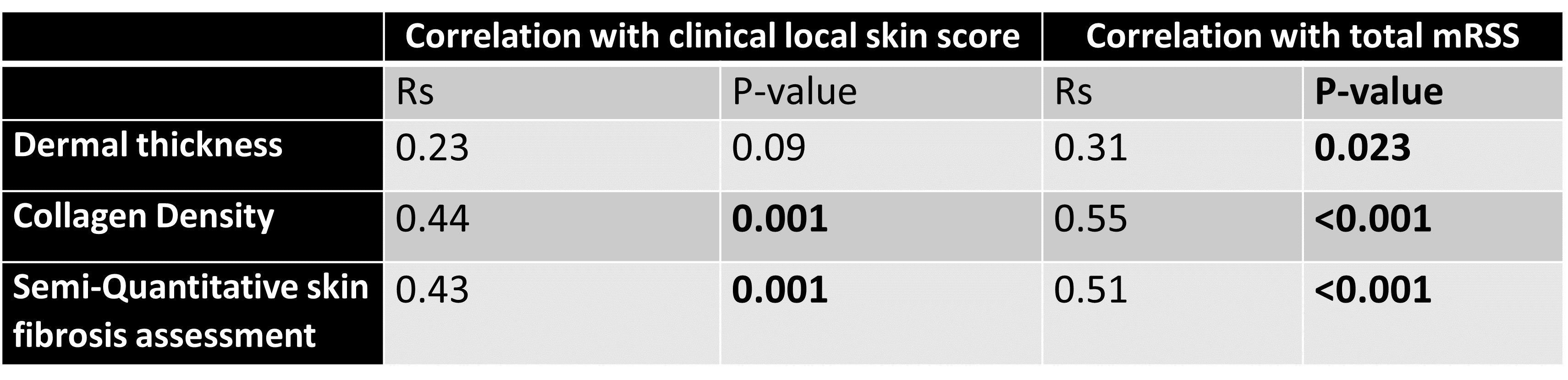
Results: The clinical local skin score at the biopsy site highly correlated with the overall mRSS (Rs=0.85, p< 0.001). As shown in Table 1, it also correlated significantly with histological collagen density and semi-quantitative fibrosis score but not with histological dermal thickness. Similarly, total mRSS showed stronger correlations with collagen density and fibrosis scoring than with histological dermal thickness.
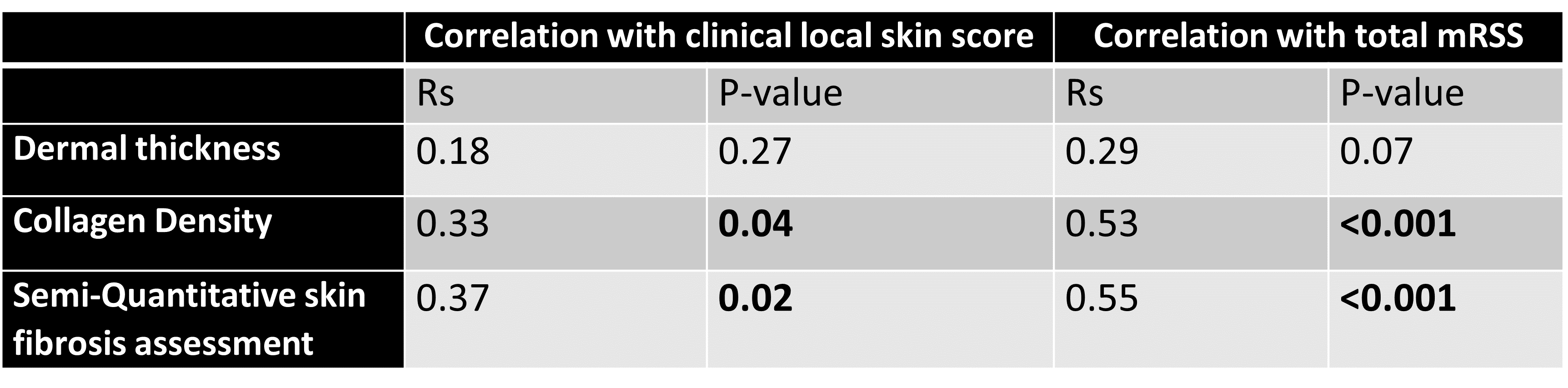
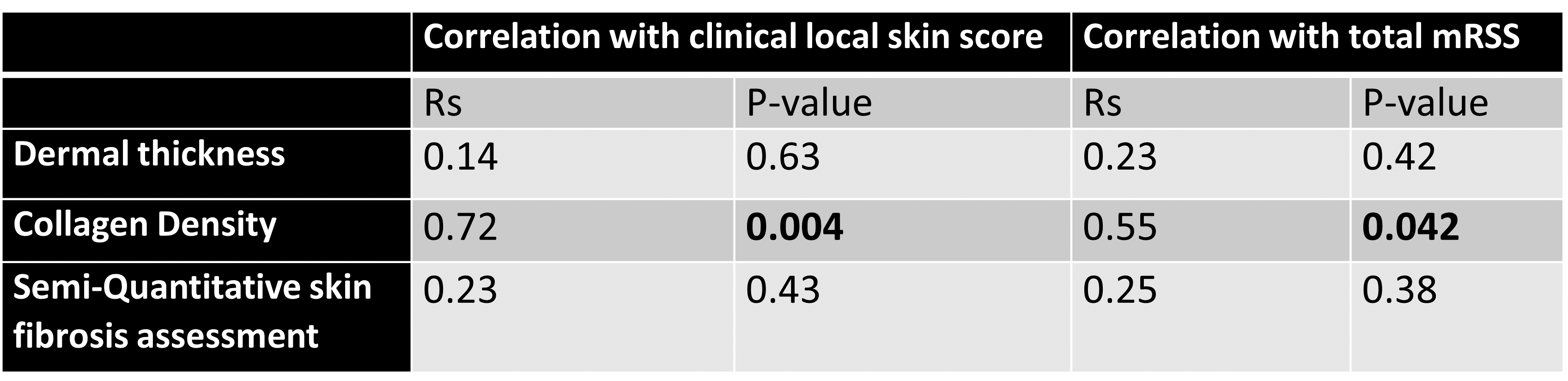
Tables 2 and 3 show the correlation of local clinical score/total mRSS with skin histology measures in diffuse and limited subtypes, respectively. Consistent with the aforementioned results, the local skin score and total mRSS did not correlate significantly with the histological dermal thickness in diffuse or limited subsets, while they correlated with the collagen density in both clinical subsets.
Conclusion: Local skin score and mRSS show moderate criterion validity with histological collagen density but poorer correlation with histological dermal thickness in the overall SSc group and its diffuse and limited subsets. True to the literal meaning of the word scleroderma (scleros=hard and derma=skin), mRSS assessment method reflects better skin induration (hardening) than skin thickening.
To cite this abstract in AMA style:
Assassi S, Desai R, Browning J, Theodore S, Zhang M, Skaug B, Alonso J, McMahan Z, Mayes M, Wu M. Criterion Validity of Modified Rodnan Skin Score: Does It Capture Skin Thickness or Hardening? Results of a Large Skin Histology Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/criterion-validity-of-modified-rodnan-skin-score-does-it-capture-skin-thickness-or-hardening-results-of-a-large-skin-histology-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/criterion-validity-of-modified-rodnan-skin-score-does-it-capture-skin-thickness-or-hardening-results-of-a-large-skin-histology-study/