Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Craniofacial localized scleroderma (LS) can lead to disfigurement and severe extracutaneous manifestations (ECMs). There is an ongoing need to standardize multidisciplinary evaluation and care. In this retrospective cohort, we report the multidisciplinary assessment and treatment over ten years at one center for 77 patients.
Methods: A retrospective Institutional Review Board-approved chart review was performed on 393 patients diagnosed with LS at Texas Childrens Hospital from January 1, 2012, to October 26, 2022. Patient inclusion criteria were as follows: 1) age < 18 at clinical diagnosis; 2) LS diagnosis using the Padua classification criteria; 3) confirmation of diagnosis by a pediatric rheumatologist, dermatologist, or plastic surgeon; 4) LS lesion involving the craniofacial area. Patients were excluded if any of the above criteria were not met as well as miscoding or insufficient medical records (Figure 1).
Results: Demographic and clinical information was collected for 77 patients who met the inclusion criteria. The mean age of diagnosis was 9 (range: 2-17) years. Most patients were female (64%) and Hispanic (55%). Diagnoses included linear scleroderma of the face/scalp (39%), Parry Romberg syndrome (21%), both (18%), mixed morphea (17%), and circumscribed morphea (5%). In most cases, a pediatric dermatologist (66.2%) made the diagnosis; 28 patients (36%) had a skin biopsy to confirm the clinical diagnosis. In 23% of patient charts, providers noted that the lesions would “burn out” or appear “burnt out.” 27% of patients had a recurrence. Patients had a documented evaluation by the following pediatric specialists: dermatology (84%), rheumatology (78%), ophthalmology (65%), dental (56%), and plastic surgery (47%). Of the 58 patients (75%) with an MRI brain or face, 41 had abnormal findings. ECMs included neurological (34%), psychological (34%), dental (29%), ophthalmological (20%), and musculoskeletal (14%) (Table 1). There were 55 patients (71%) on systemic medications including corticosteroids (76.4%), methotrexate (96.4%), mycophenolate mofetil (16.4%), biologics (7.3%), and other medications (hydroxychloroquine or intravenous immunoglobulin, 36.4%). Twenty-four patients (31%) have undergone surgery at a mean age of 13 (range: 5-18). The most common surgical procedure was an autologous fat graft (n =22) followed by rhinoplasty (n=2), eye repair (n=1), flap (n=1), hyaluronic filler (n=1), implant (n=1), and osteotomy (n=1). The mean number of fat grafts per patient was 2 (range: 1-7). The only surgical complications noted were a poor cosmetic outcome for the flap procedure and chronic infection of the malar implant leading to removal. Death occurred in one patient secondary to status epilepticus.
Conclusion: Despite published evaluation algorithms, there are still gaps in comprehensive multidisciplinary care for craniofacial LS. There needs to be improved collaboration with ophthalmology, dental, plastic surgery, and psychology. Topical treatment alone is inadequate, so referral to pediatric rheumatology for systemic immunosuppressant treatment is critical. Given the risk of recurrence, ongoing monitoring is essential.
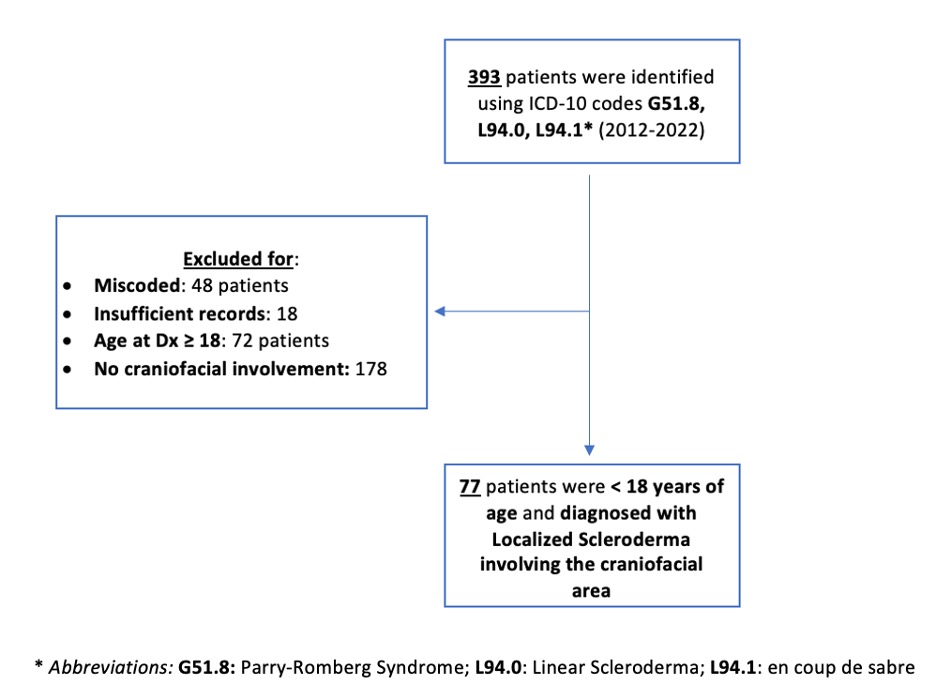 Figure 1. Retrospective cohort study design and flow chart.
Figure 1. Retrospective cohort study design and flow chart.
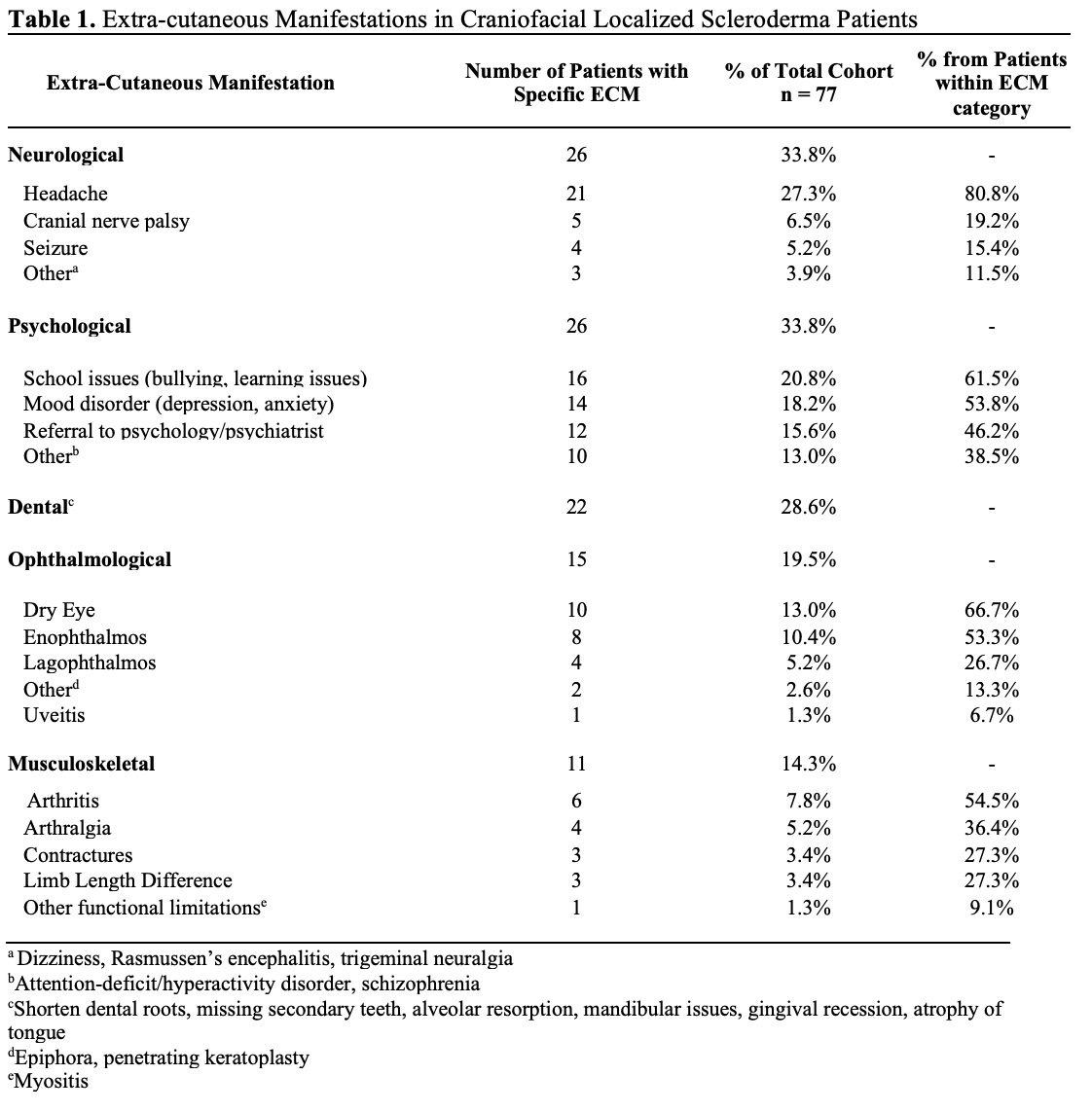 Table 1. Extra-cutaneous manifestations in craniofacial localized scleroderma patients.
Table 1. Extra-cutaneous manifestations in craniofacial localized scleroderma patients.
To cite this abstract in AMA style:
Stubbs L, Hashemi A, Hunt R, Maricevich R. Craniofacial Localized Scleroderma: A Single Center Retrospective Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/craniofacial-localized-scleroderma-a-single-center-retrospective-cohort/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/craniofacial-localized-scleroderma-a-single-center-retrospective-cohort/
