Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: We previously surveyed individuals with systemic sclerosis (SSc) enrolled in Scleroderma Patient-centered Intervention Network (SPIN) Cohort between April-May 2021 to determine COVID-19 vaccination rates, adverse reactions, and vaccine hesitancy. The purpose of the present study was to extend analyses through June-July 2022 and assess (1) COVID vaccination rates, including boosters; (2) vaccine-related adverse events from primary series and booster vaccine doses; (3) peri-vaccination immunosuppressive (IS) medication management; (4) vaccine hesitancy and perceptions; and (5) rate and severity of self-reported COVID-19 in SSc.
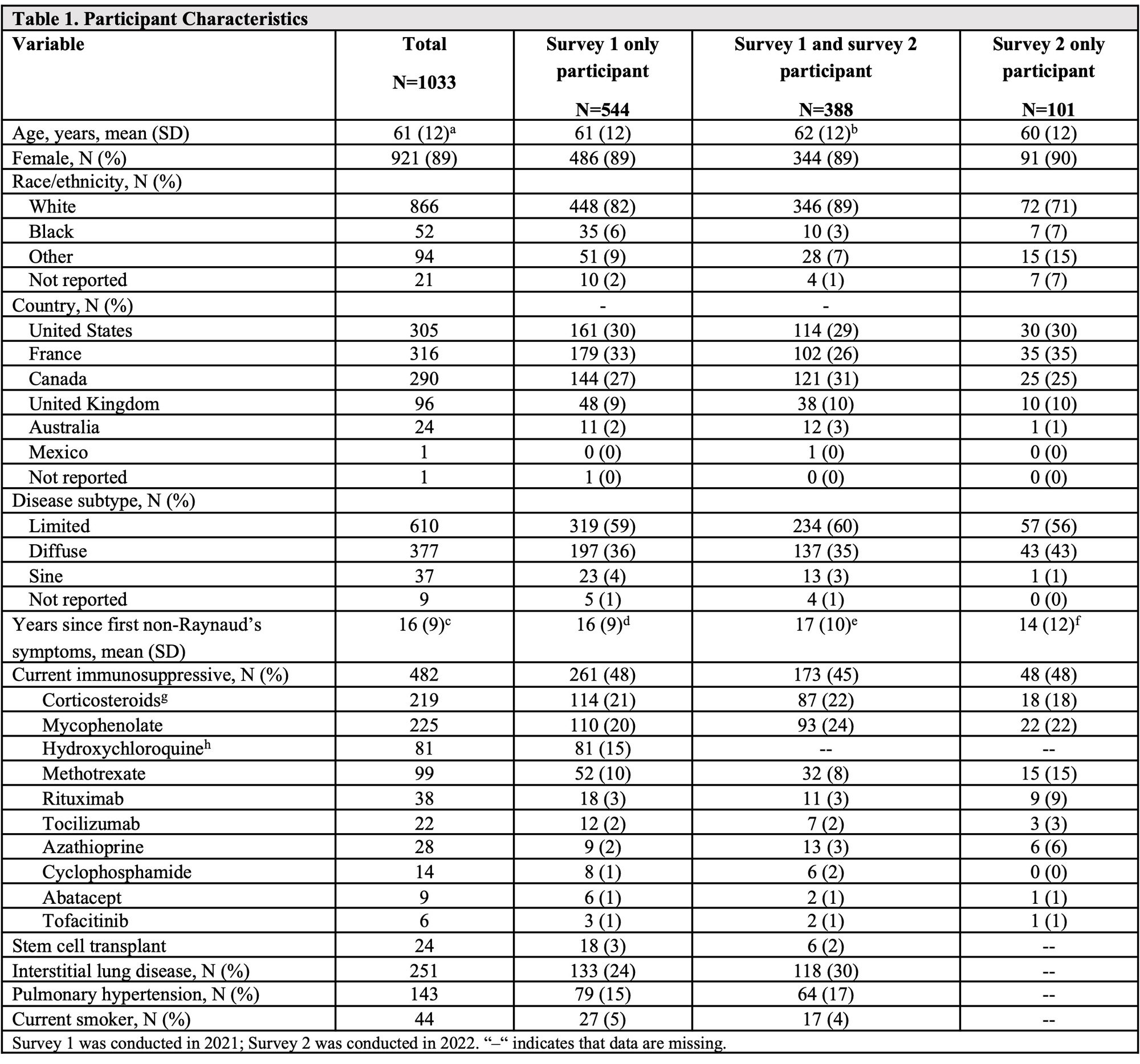
Methods: Participants were adults with SSc enrolled in the SPIN Cohort, which includes 47 centers from seven countries. Participants from the SPIN Cohort, including those who did and did not complete the 2021 survey, were invited to complete the second survey from June 1 to July 4, 2022. The survey included items related to COVID-19 history, COVID-19 vaccinations, and perceptions related to COVID-19 vaccines. Participants were considered “fully vaccinated” if they had completed a primary vaccine series and received at least one booster dose.
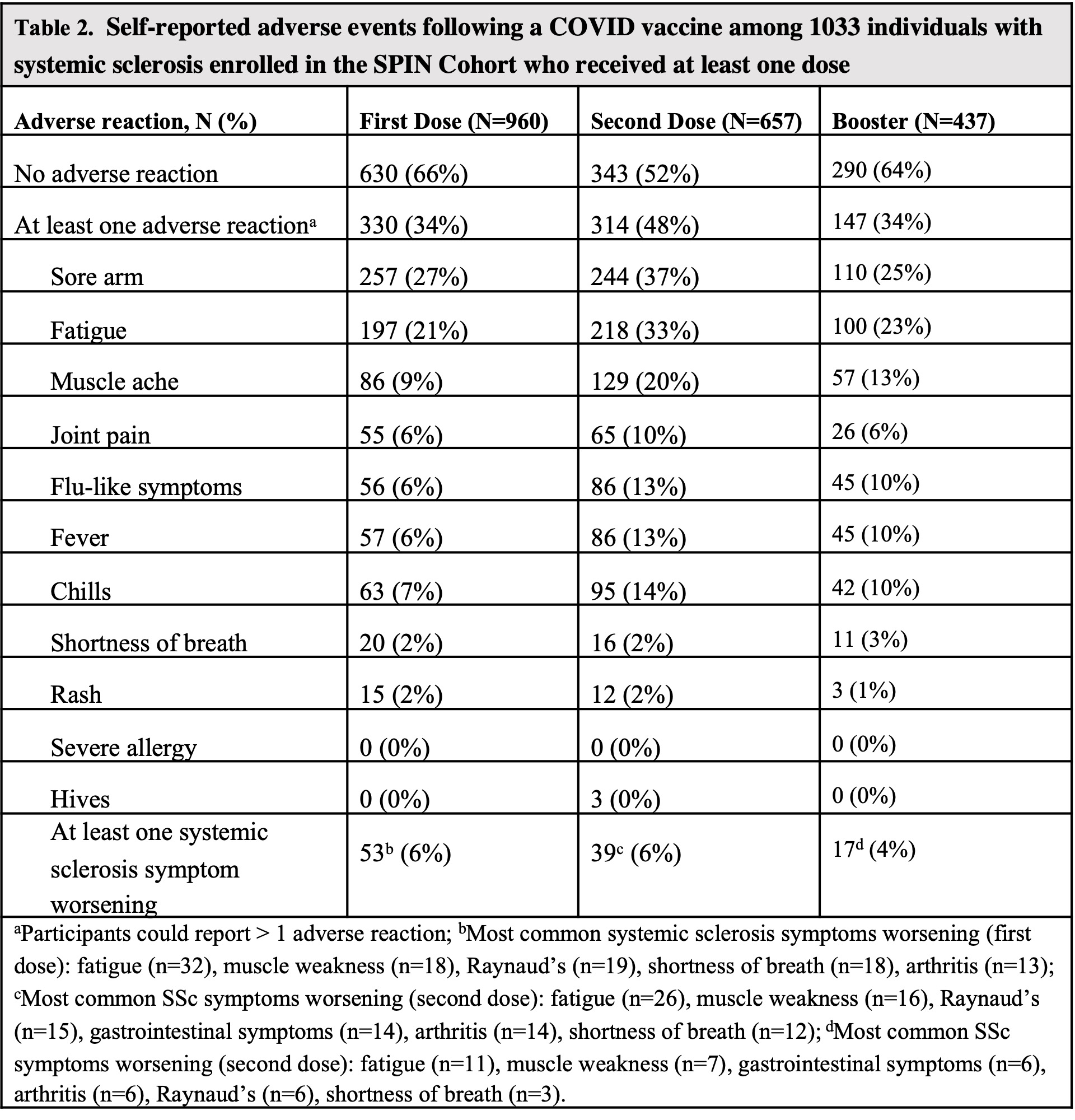
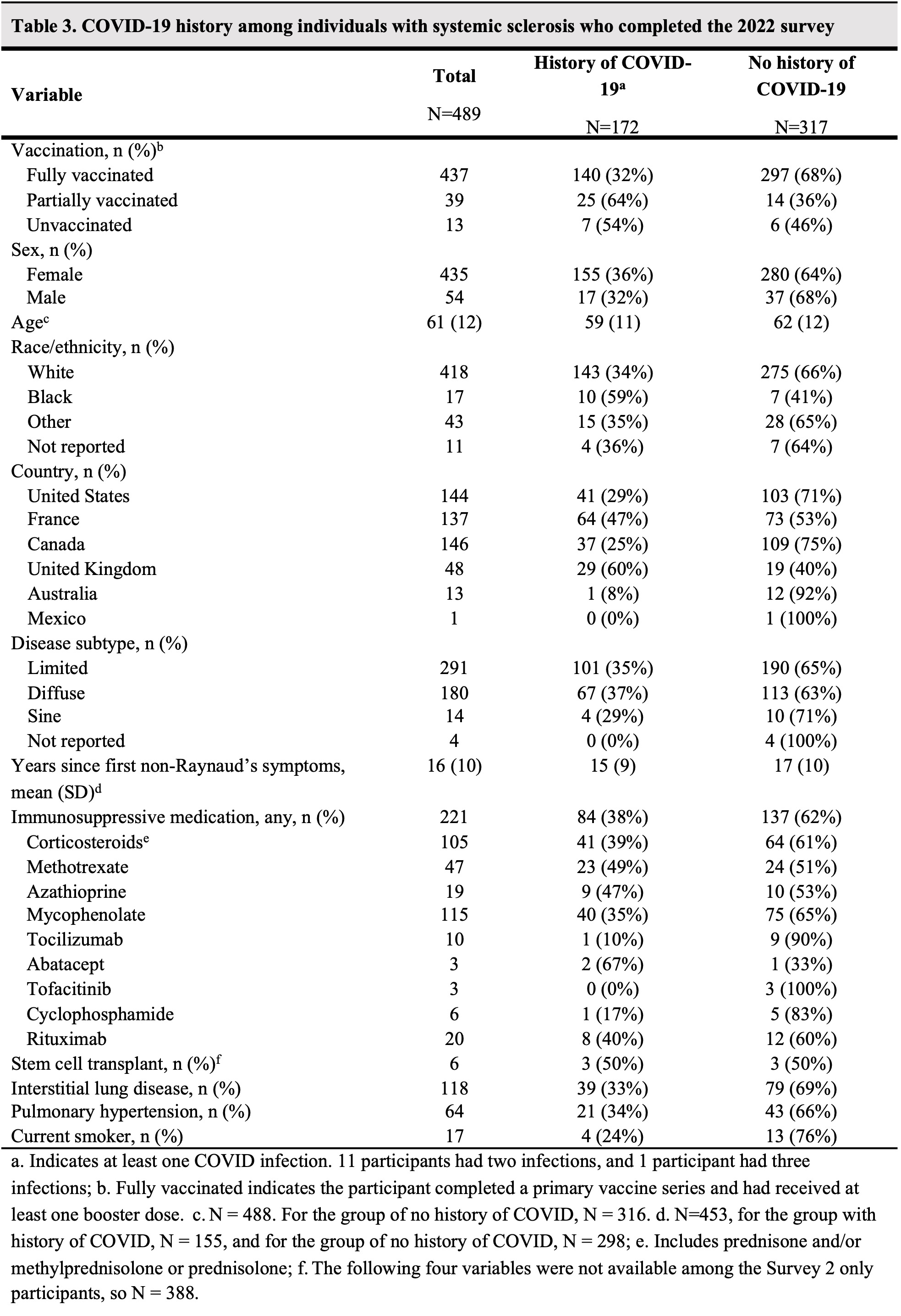
Results: 544 participants answered the 2021 survey only, 101 answered the 2022 survey only, and 388 answered both. Participant characteristics are shown in Table 1. 437 of 489 (89%) participants who completed the 2022 survey received a booster dose. 18 of 31 (58%) participants who were vaccine hesitant in 2021 were vaccinated by the 2022 survey. Adverse events following primary vaccine and booster doses are shown in Table 2. SSc symptom worsening was reported after the first and second vaccine dose in 6% (53 of 960 and 39 of 657, respectively) and after the booster dose in 4% (17 of 437) (Table 2). Of 157 patients taking methotrexate (MTX) or mycophenolate (MMF), 129 (82%) held those at the time of booster vaccination. Of 58 individuals not fully vaccinated, 29 (50%) were worried that the vaccine would cause an SSc flare; 19 (33%) indicated that they did not have enough information to decide about vaccination. 172 of 489 (35%) from the 2022 survey reported a history of COVID-19 (Table 3), 114 (66%) of which occurred after completing a primary vaccine series. 9 (5%) were asymptomatic, 66 (44%) had mild symptoms, 82 (48%) had moderate symptoms, and 15 (9%) required hospitalization. Of those 15 hospitalized individuals, 9 (60%) were taking at least one IS medication, 11 were fully vaccinated, 3 were partially vaccinated, and 1 was unvaccinated at the time of hospitalization.
Conclusion: Most participants were fully vaccinated, and a majority held their MTX or MMF post-booster vaccination, which is aligned with recent American College of Rheumatology Guidance. Most vaccine-hesitant participants were concerned regarding risk of SSc flare; however, few vaccinated participants reported an SSc flare following primary (6%) or booster doses (4%). In a highly vaccinated cohort, 35% reported a history of COVID-19, of whom 9% required hospital care. This is the largest, longitudinal study to-date of COVID-19 focused upon SSc. These data may be used to counsel patients regarding vaccine safety and COVID-19 outcomes.
To cite this abstract in AMA style:
Lakin K, Gordon J, Wu Y, Kwakkenbos L, Carrier M, Henry R, Denton C, Mouthon L, Spiera R, Thombs B. COVID Vaccinations and Infections Among Individuals with Systemic Sclerosis: A Scleroderma Patient-centered Intervention Network (SPIN) Cohort Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/covid-vaccinations-and-infections-among-individuals-with-systemic-sclerosis-a-scleroderma-patient-centered-intervention-network-spin-cohort-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-vaccinations-and-infections-among-individuals-with-systemic-sclerosis-a-scleroderma-patient-centered-intervention-network-spin-cohort-study/