Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: We have previously shown that short term progression of interstitial lung disease (ILD) in systemic sclerosis (SSc) is associated with mortality. However, it is less clear how multiple progressive ILD events over years affect mortality. The objective was to determine the number of progressive SSc-ILD events over three years and assess its impact on mortality.
Methods: We included all SSc patients from two expert SSc centers with well characterized SSc cohorts who had ILD on HRCT, consecutive annual lung functions including forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) and comprehensive serial clinical and imaging assessments available. ILD progressive events were observed over a follow-up period of 3 years segregated in no, 1 or >1 progressive event using:
(A) FVC ≥5% decline over 12 months
(B) 2022 ATS/ERS/JRS/ALAT guideline progressive pulmonary fibrosis (PPF) criteria with (1) worsening of respiratory symptoms; (2) absolute decline in FVC ≥5% or in DLCO ≥10% and (3) disease progression on HRCT over 12 months
(C) INBUILD progressive fibrosing ILD (PF-ILD) criteria with (1) relative FVC decline ≥10%, (2) relative FVC decline ≥5-< 10% and worsening of respiratory symptoms or an increased extent of fibrosis on HRCT, or (3) worsening of respiratory symptoms and an increased extent of fibrosis within 24 months
(D) Composite decline with FVC ≥10% decline; or FVC ≥5%-9% and DLCO ≥15% over 12 months
Survival was assessed with Kaplan-Meier survival estimates. Multivariable Cox regression was applied, adjusting for known risk factors including treatment to identify the impact of multiple progressive ILD events on mortality.
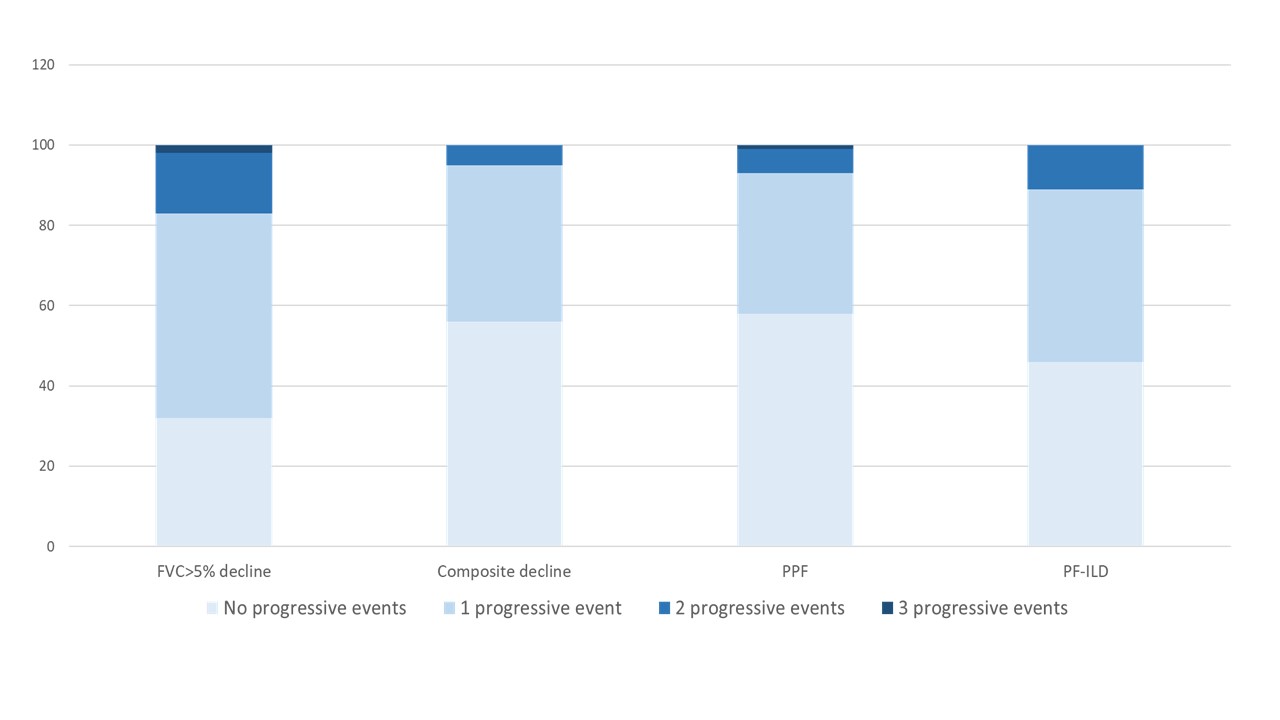
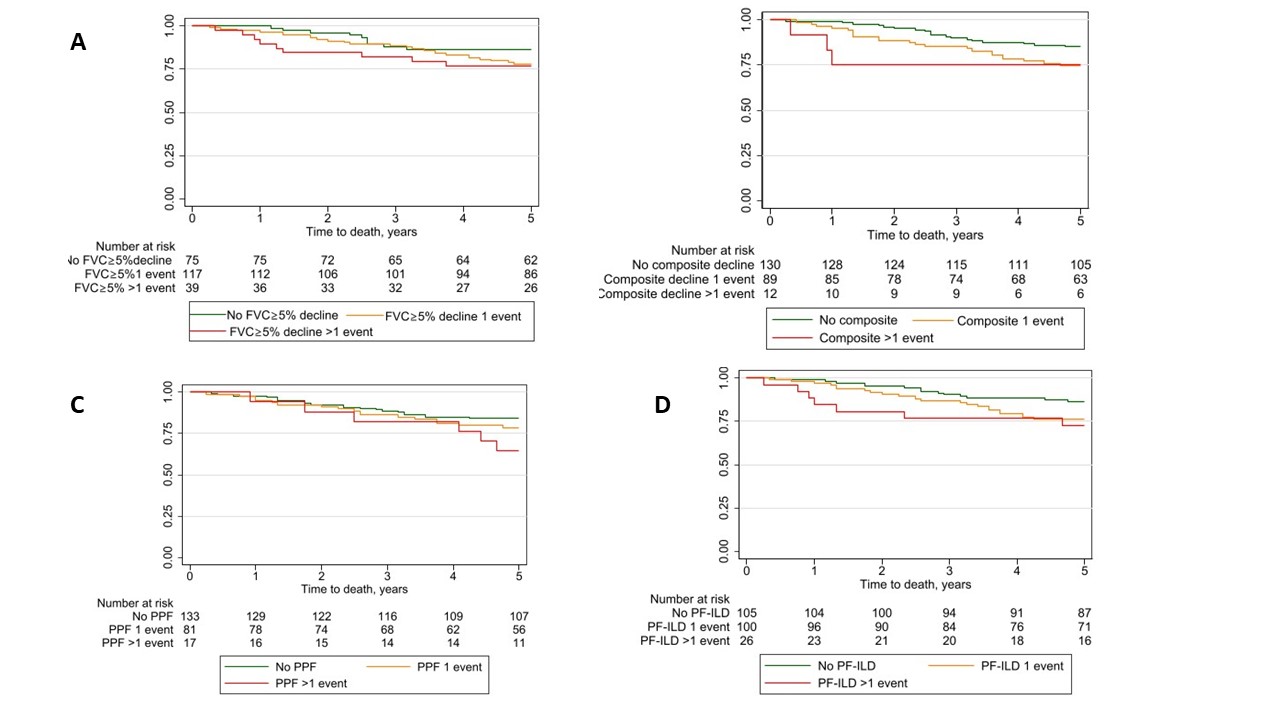
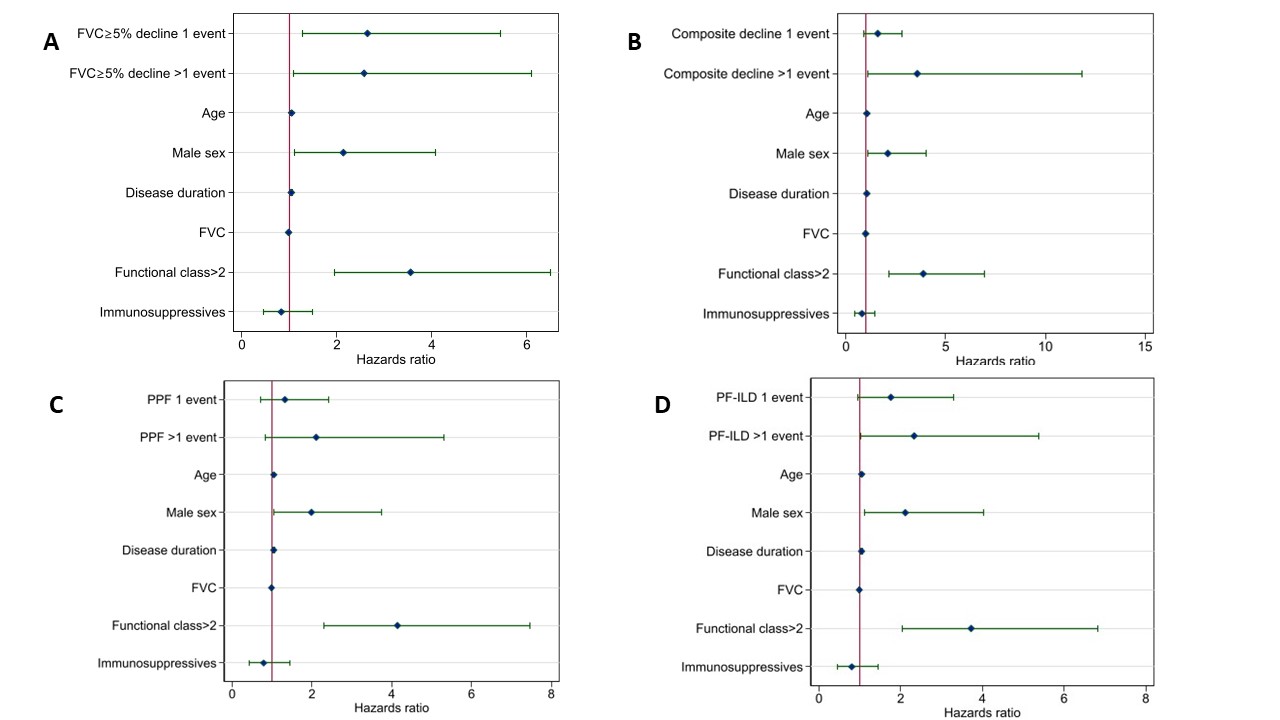
Results: In total, 231 SSc-ILD patients were included. The number of progressive events varied depending on the applied definition, with FVC ≥5% decline showing >1 event over 3 years in 17%, compared to 5% using composite decline, 7% using PPF and 11% using the PF-ILD criteria (Figure 1). Over the observation period of 7.7 (SD3.9) years, 81 (35%) died. When assessing the impact of number of events on survival by Kaplan Meier estimates, we found significant difference for FVC ≥5% decline, composite decline, PF-ILD but not PPF (Figure 2).When assessing the impact of number of progressive events on mortality compared to no progression adjusted for other known risk factors, we identified that >1 events of PF-ILD and composite decline were associated with increased mortality (Figure 3).
Conclusion: ILD progression has a major impact on long-term outcome with even one event of FVC≥5% decline reducing long-term survival, but with multiple events further reducing survival. It is therefore of high importance to prevent progression in SSc-ILD to improve survival.
To cite this abstract in AMA style:
Hoffmann-Vold A, Petelytska L, Fretheim H, Aaløkken T, Becker M, Brunborg C, Bruni C, Clarenbach C, Diep P, Dobrota R, Durheim M, Elhai M, Frauenfelder T, Jordan S, Langballe E, Midtvedt O, Mihai C, Molberg O, Distler O. Continuous Progressive ILD in Systemic Sclerosis Is Associated with Mortality [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/continuous-progressive-ild-in-systemic-sclerosis-is-associated-with-mortality/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continuous-progressive-ild-in-systemic-sclerosis-is-associated-with-mortality/