Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies are systemic inflammatory conditions characterized by muscle weakness and reduced muscle endurance that limit activities of daily living. Daily step count is an accepted metric of physical activity used in many population studies. Wearable technologies enable tracking of daily step counts longitudinally. The Actigraph is an FDA-approved triaxial accelerometer and tracks step count with high accuracy. It is used for research and not commercially available. Fitbit also measures step counts and is the most widely used consumer-based activity tracker with nearly 30 million users and many studies have validated Fitbit step counts compared to accelerometers. Given the need for objective measures of physical activity in myositis patients and broad availability of Fitbit, we assessed the reliability, validity and responsiveness of Fitbit in evaluating physical function in myositis patients and compared the accuracy of Fitbit step counts to the Actigraph.
Methods: This was a prospective longitudinal observational study with 3-visits at 0, 3-, and 6-months in polymyositis (PM), dermatomyositis (DM), necrotizing myopathy (NM) or anti-synthetase syndrome (AS) subjects. Six myositis core set measures [manual muscle testing (MMT), physician global disease activity (MD-GDA), patient global disease activity (PT-GDA), extra-muscular global disease activity (EM-GDA), HAQ-DI and creatine kinase (CK)], three functional measures [six-minute walk (6MWD), timed up-and-go (TUG) and sit-to-stand tests (STS)] and SF-36 physical function-10 (PF10) were collected at each visit. Total improvement score (TIS) was used as a measure of clinical response. Patients used a waist-worn Fitbit One and ActiGraph T3X-BT concurrently for 7 days/month for 6 consecutive months with a “valid day” defined as requiring ≥1500 steps.
Results: Twenty-four (10 DM, 8 PM/NM and 6 AS without myositis) patients (17 females/7 males; 91% Caucasian) were enrolled. Test-retest reliability of daily step count was strong in patients who had 1-month follow-up (r:0.84, ICC 0.89). Daily step count showed moderate-strong correlations with MD-GDA, PT-GDA, HAQ-DI, SF-36 PF10 and all three functional tests supporting convergent validity (Table 1). Patients with inactive muscle disease had 49% higher daily step count than patients with active myositis. Fitbit and Actigraph demonstrated excellent agreement and strong correlation (Rho:0.97, ICC 0.96; Figure 1). Fitbit overestimated the daily step count in two-thirds of the patients with a median of 6.8% (3.8-14.6). The absolute change in daily steps at 6 months correlated moderately-strongly with absolute changes in MD-GDA, TIS and 6MWD.
Conclusion: Fitbit measured daily step counts are a reliable and valid measure of physical activity in a cohort of myositis patients. Daily step counts measured by Fitbit and Actigraph are in strong agreement with a slight overestimation by Fitbit. This pilot data suggests that Fitbit daily step counts have a potential to be used in clinical practice and trials to monitor physical activity in myositis patients, but larger studies are needed for further validation.
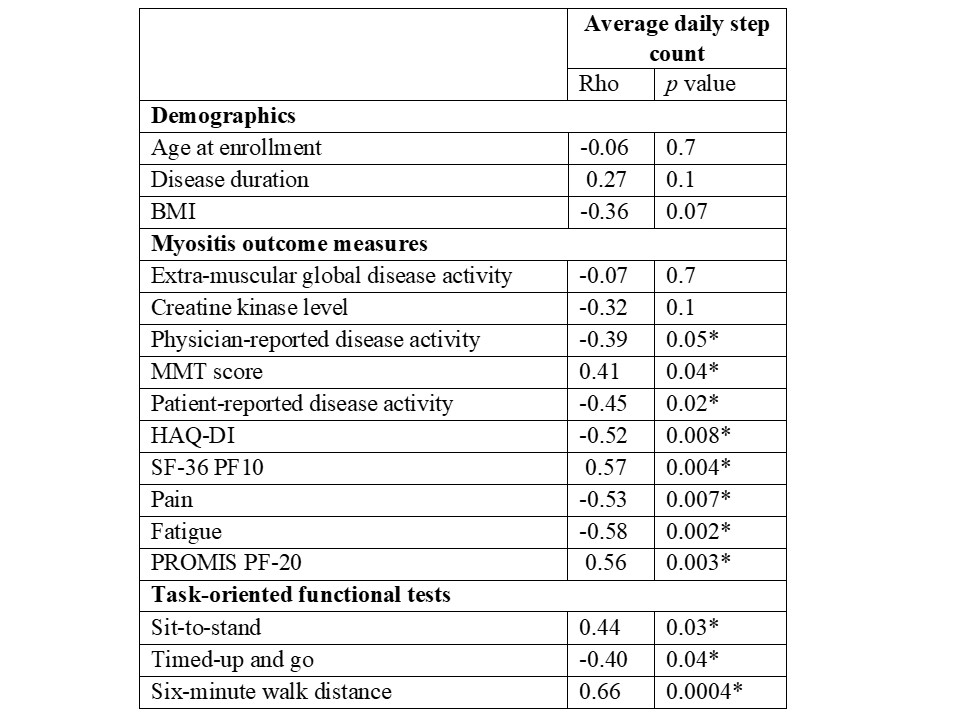 Correlations of Fitbit average daily steps and demographics, myositis outcome measures, and task-oriented functional tests.
Correlations of Fitbit average daily steps and demographics, myositis outcome measures, and task-oriented functional tests.
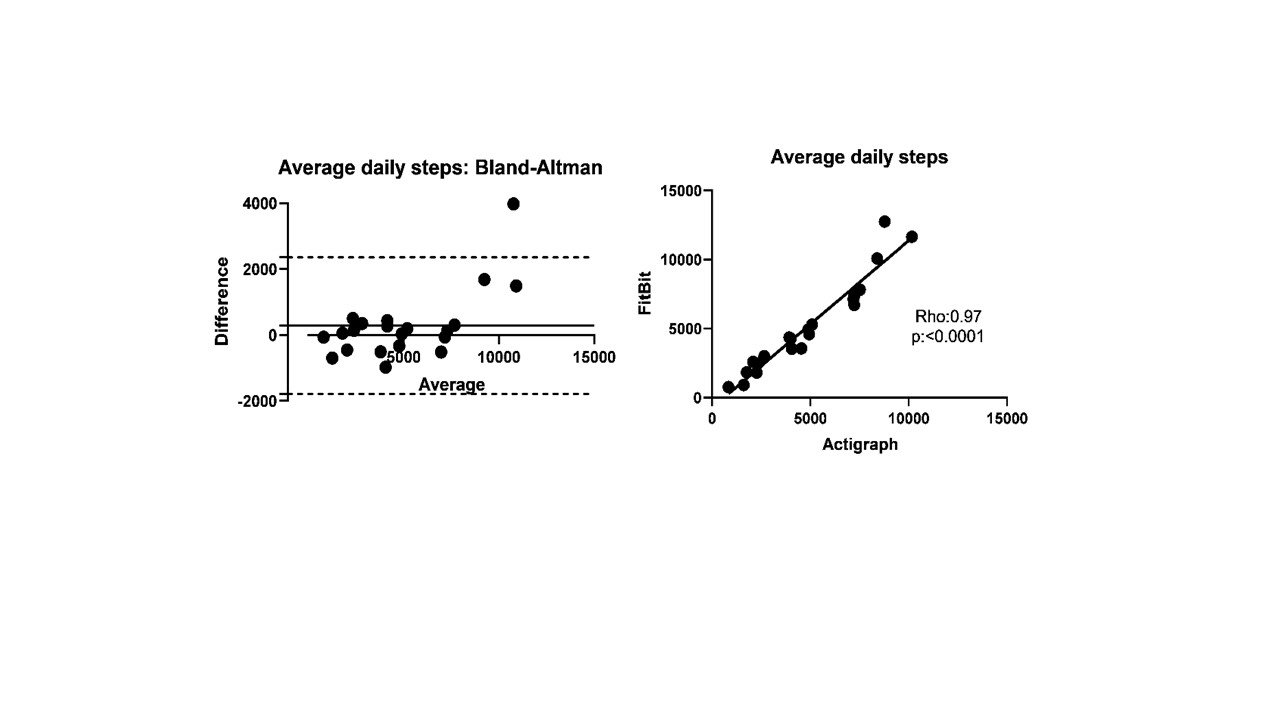 Agreement (Bland-Altman plot) and correlation (scatter plot) between FitBit and Actigraph measured average daily steps in patients wearing both devices at the same time for the same duration.
Agreement (Bland-Altman plot) and correlation (scatter plot) between FitBit and Actigraph measured average daily steps in patients wearing both devices at the same time for the same duration.
To cite this abstract in AMA style:
Saygin D, Rockette-Wagner B, Oddis C, Koontz D, Moghadam-Kia S, Aggarwal R. Consumer-based Activity Trackers in Evaluation of Physical Function in Myositis Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/consumer-based-activity-trackers-in-evaluation-of-physical-function-in-myositis-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consumer-based-activity-trackers-in-evaluation-of-physical-function-in-myositis-patients/
